Sphingolipids in lung endothelial biology and regulation of vascular integrity
- PMID: 23563658
- PMCID: PMC7706350
- DOI: 10.1007/978-3-7091-1511-4_10
Sphingolipids in lung endothelial biology and regulation of vascular integrity
Abstract
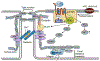
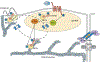
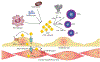
Of the multiple and diverse homeostatic events that involve the lung vascular endothelium, participation in preserving vascular integrity and therefore organ function is paramount. We were the first to show that the lipid growth factor and angiogenic factor, sphingosine-1-phosphate, is a critical agonist involved in regulation of human lung vascular barrier function (Garcia et al. J Clin Invest, 2011). Utilizing both in vitro models and preclinical murine, rat, and canine models of acute and chronic inflammatory lung injury, we have shown that S1Ps, as well as multiple S1P analogues such as FTY720 and ftysiponate, serve as protective agents limiting the disruption of the vascular EC monolayer in the pulmonary microcirculation and attenuate parenchymal accumulation of inflammatory cells and high protein containing extravasated fluid, thereby reducing interstitial and alveolar edema. The vasculo-protective mechanism of these therapeutic effects occurs via ligation of specific G-protein-coupled receptors and an intricate interplay of S1P with other factors (such as MAPKS, ROCKs, Rho, Rac1) with rearrangement of the endothelial cytoskeleton to form strong cortical actin rings in the cell periphery and enhanced cell-to-cell and cell-to-matrix tethering dynamics. This cascade leads to reinforcement of focal adhesions and paracellular junctional complexes via cadherin, paxillin, catenins, and zona occludens. S1P through its interaction with Rac and Rho influences the cytoskeletal rearrangement indicated in the later stages of angiogenesis as a stabilizing force, preventing excessive vascular permeability. These properties translate into a therapeutic potential for acute and chronic inflammatory lung injuries. S1P has potential for providing a paradigm shift in the approach to disruption of critical endothelial gatekeeper function, loss of lung vascular integrity, and increased vascular permeability, defining features of acute lung injury (ALI), and may prove to exhibit an intrinsically protective role in the pulmonary vasculature ameliorating agonist- or sepsis-induced pulmonary injury and vascular leakage.
Figures
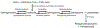
References
-
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K (1996) Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271(34):20246–20249 - PubMed
-
- An S, Zheng Y, Bleu T (2000) Sphingosine 1-phosphate-induced cell proliferation, survival, and related signaling events mediated by G protein-coupled receptors Edg3 and Edg5. J Biol Chem 275(1):288–296 - PubMed
-
- Argraves KM, Wilkerson BA, Argraves WS, Fleming PA, Obeid LM, Drake CJ (2004) Sphingosine-1-phosphate signaling promotes critical migratory events in vasculogenesis. J Biol Chem 279(48): 50580–50590 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

