Cadherin mechanotransduction in tissue remodeling
- PMID: 23563964
- PMCID: PMC11113614
- DOI: 10.1007/s00018-013-1329-x
Cadherin mechanotransduction in tissue remodeling
Abstract
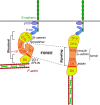
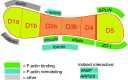
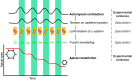
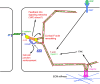
Mechanical forces are increasingly recognized as central factors in the regulation of tissue morphogenesis and homeostasis. Central to the transduction of mechanical information into biochemical signaling is the contractile actomyosin cytoskeleton. Fluctuations in actomyosin contraction are sensed by tension sensitive systems at the interface between actomyosin and cell adhesion complexes. We review the current knowledge about the mechanical coupling of cell-cell junctions to the cytoskeleton and highlight the central role of α-catenin in this linkage. We assemble current knowledge about α-catenin's regulation by tension and about its interactions with a diversity of proteins. We present a model in which α-catenin is a force-regulated platform for a machinery of proteins that orchestrates local cortical remodeling in response to force. Finally, we highlight recently described fundamental processes in tissue morphogenesis and argue where and how this α-catenin-dependent cadherin mechanotransduction may be involved.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

