HER2-positive gastric cancer
- PMID: 23563986
- PMCID: PMC3889288
- DOI: 10.1007/s10120-013-0252-z
HER2-positive gastric cancer
Abstract
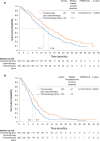
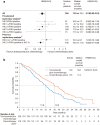
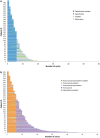
Human epidermal growth factor receptor 2 (HER2) is involved in the pathogenesis and poor outcomes of several types of cancer, including advanced gastric and gastroesophageal junction cancer. Molecular-targeted drugs, such as trastuzumab, which prolong overall survival and progression-free survival in HER2-positive breast cancer, may also be beneficial in patients with HER2-positive gastric cancer. Several studies have examined this possibility, such as the Trastuzumab for Gastric Cancer trial. In this context, the first part of this review provides an update on our knowledge of HER2 in breast and gastric cancer, including the detection and prognostic relevance of HER2 in gastric cancer. The second part of the review discusses the results of pivotal clinical trials that examined the potential for using trastuzumab to treat this disease. This section also summarizes the trials that have been conducted or that are underway to determine the optimal uses of trastuzumab in gastric cancer, including its use as monotherapy and continuation beyond disease progression. The final section discusses the future prospects of other anti-HER2 drugs, including lapatinib, trastuzumab emtansine, and pertuzumab, for the treatment of HER2-positive gastric cancer. The introduction of trastuzumab led to the establishment of a new disease entity, "HER2-positive gastric cancer," similar to HER2-positive breast cancer. It is expected that more anti-HER2 drugs will be developed and introduced into clinical practice to treat HER2-positive cancers, including gastric cancer.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

