Energetics and location of phosphoinositide binding in human Kir2.1 channels
- PMID: 23564459
- PMCID: PMC3675606
- DOI: 10.1074/jbc.M113.452540
Energetics and location of phosphoinositide binding in human Kir2.1 channels
Abstract
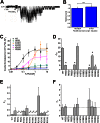
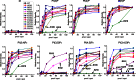
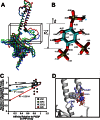
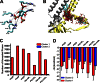
Kir2.1 channels are uniquely activated by phosphoinositide 4,5-bisphosphate (PI(4,5)P2) and can be inhibited by other phosphoinositides (PIPs). Using biochemical and computational approaches, we assess PIP-channel interactions and distinguish residues that are energetically critical for binding from those that alter PIP sensitivity by shifting the open-closed equilibrium. Intriguingly, binding of each PIP is disrupted by a different subset of mutations. In silico ligand docking indicates that PIPs bind to two sites. The second minor site may correspond to the secondary anionic phospholipid site required for channel activation. However, 96-99% of PIP binding localizes to the first cluster, which corresponds to the general PI(4,5)P2 binding location in recent Kir crystal structures. PIPs can encompass multiple orientations; each di- and triphosphorylated species binds with comparable energies and is favored over monophosphorylated PIPs. The data suggest that selective activation by PI(4,5)P2 involves orientational specificity and that other PIPs inhibit this activation through direct competition.
Keywords: Lipids; Molecular Docking; Molecular Modeling; Phosphatidylinositol Phosphatase; Potassium Channels.
Figures



References
-
- Enkvetchakul D., Jeliazkova I., Nichols C. G. (2005) Direct modulation of Kir channel gating by membrane phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 280, 35785–35788 - PubMed
-
- Leal-Pinto E., Gómez-Llorente Y., Sundaram S., Tang Q. Y., Ivanova-Nikolova T., Mahajan R., Baki L., Zhang Z., Chavez J., Ubarretxena-Belandia I., Logothetis D. E. (2010) Gating of a G protein-sensitive mammalian Kir3.1 prokaryotic Kir channel chimera in planar lipid bilayers. J. Biol. Chem. 285, 39790–39800 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

