Calreticulin regulates transforming growth factor-β-stimulated extracellular matrix production
- PMID: 23564462
- PMCID: PMC3656311
- DOI: 10.1074/jbc.M112.447243
Calreticulin regulates transforming growth factor-β-stimulated extracellular matrix production
Abstract
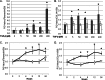
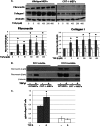
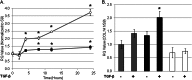
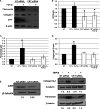
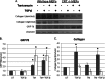
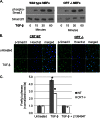
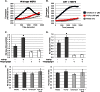
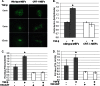
Endoplasmic reticulum (ER) stress is an emerging factor in fibrotic disease, although precise mechanisms are not clear. Calreticulin (CRT) is an ER chaperone and regulator of Ca(2+) signaling up-regulated by ER stress and in fibrotic tissues. Previously, we showed that ER CRT regulates type I collagen transcript, trafficking, secretion, and processing into the extracellular matrix (ECM). To determine the role of CRT in ECM regulation under fibrotic conditions, we asked whether CRT modified cellular responses to the pro-fibrotic cytokine, TGF-β. These studies show that CRT-/- mouse embryonic fibroblasts (MEFs) and rat and human idiopathic pulmonary fibrosis lung fibroblasts with siRNA CRT knockdown had impaired TGF-β stimulation of type I collagen and fibronectin. In contrast, fibroblasts with increased CRT expression had enhanced responses to TGF-β. The lack of CRT does not impact canonical TGF-β signaling as TGF-β was able to stimulate Smad reporter activity in CRT-/- MEFs. CRT regulation of TGF-β-stimulated Ca(2+) signaling is important for induction of ECM. CRT-/- MEFs failed to increase intracellular Ca(2+) levels in response to TGF-β. NFAT activity is required for ECM stimulation by TGF-β. In CRT-/- MEFs, TGF-β stimulation of NFAT nuclear translocation and reporter activity is impaired. Importantly, CRT is required for TGF-β stimulation of ECM under conditions of ER stress, as tunicamycin-induced ER stress was insufficient to induce ECM production in TGF-β stimulated CRT-/- MEFs. Together, these data identify CRT-regulated Ca(2+)-dependent pathways as a critical molecular link between ER stress and TGF-β fibrotic signaling.
Keywords: Calcium; Calreticulin; Collagen; Endoplasmic Reticulum Stress; Fibronectin; Fibrosis; NFAT Transcription Factor; Transforming Growth Factor β (TGFβ).
Figures

Similar articles
-
Calreticulin is important for the development of renal fibrosis and dysfunction in diabetic nephropathy.Matrix Biol Plus. 2020 Apr 3;8:100034. doi: 10.1016/j.mbplus.2020.100034. eCollection 2020 Nov. Matrix Biol Plus. 2020. PMID: 33543033 Free PMC article.
-
Intracellular calreticulin regulates multiple steps in fibrillar collagen expression, trafficking, and processing into the extracellular matrix.J Biol Chem. 2010 Mar 5;285(10):7067-78. doi: 10.1074/jbc.M109.006841. Epub 2009 Dec 31. J Biol Chem. 2010. PMID: 20044481 Free PMC article.
-
Calreticulin exploits TGF-β for extracellular matrix induction engineering a tissue regenerative process.FASEB J. 2020 Dec;34(12):15849-15874. doi: 10.1096/fj.202001161R. Epub 2020 Oct 5. FASEB J. 2020. PMID: 33015849
-
The role of the endoplasmic reticulum protein calreticulin in mediating TGF-β-stimulated extracellular matrix production in fibrotic disease.J Cell Commun Signal. 2018 Mar;12(1):289-299. doi: 10.1007/s12079-017-0426-2. Epub 2017 Oct 28. J Cell Commun Signal. 2018. PMID: 29080087 Free PMC article. Review.
-
Calreticulin: non-endoplasmic reticulum functions in physiology and disease.FASEB J. 2010 Mar;24(3):665-83. doi: 10.1096/fj.09-145482. Epub 2009 Nov 25. FASEB J. 2010. PMID: 19940256 Free PMC article. Review.
Cited by
-
Efferocytosis of vascular cells in cardiovascular disease.Pharmacol Ther. 2022 Jan;229:107919. doi: 10.1016/j.pharmthera.2021.107919. Epub 2021 Jun 23. Pharmacol Ther. 2022. PMID: 34171333 Free PMC article. Review.
-
Endoplasmic reticulum in health and disease: the 12th International Calreticulin Workshop, Delphi, Greece.J Cell Mol Med. 2017 Dec;21(12):3141-3149. doi: 10.1111/jcmm.13413. Epub 2017 Nov 21. J Cell Mol Med. 2017. PMID: 29160038 Free PMC article.
-
Calreticulin is important for the development of renal fibrosis and dysfunction in diabetic nephropathy.Matrix Biol Plus. 2020 Apr 3;8:100034. doi: 10.1016/j.mbplus.2020.100034. eCollection 2020 Nov. Matrix Biol Plus. 2020. PMID: 33543033 Free PMC article.
-
Calreticulin is required for development of the cumulus oocyte complex and female fertility.Sci Rep. 2015 Sep 21;5:14254. doi: 10.1038/srep14254. Sci Rep. 2015. PMID: 26388295 Free PMC article.
-
Co-culture of human fibroblasts and Borrelia burgdorferi enhances collagen and growth factor mRNA.Arch Dermatol Res. 2018 Mar;310(2):117-126. doi: 10.1007/s00403-017-1797-1. Epub 2017 Dec 6. Arch Dermatol Res. 2018. PMID: 29214350 Free PMC article.
References
-
- Lawson W. E., Cheng D. S., Degryse A. L., Tanjore H., Polosukhin V. V., Xu X. C., Newcomb D. C., Jones B. R., Roldan J., Lane K. B., Morrisey E. E., Beers M. F., Yull F. E., Blackwell T. S. (2011) Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc. Natl. Acad. Sci. U. S. A. 108, 10562–10567 - PMC - PubMed
-
- Kypreou K. P., Kavvadas P., Karamessinis P., Peroulis M., Alberti A., Sideras P., Psarras S., Capetanaki Y., Politis P. K., Charonis A. S. (2008) Altered expression of calreticulin during the development of fibrosis. Proteomics 8, 2407–2419 - PubMed
-
- Khan M. I., Pichna B. A., Shi Y., Bowes A. J., Werstuck G. H. (2009) Evidence supporting a role for endoplasmic reticulum stress in the development of atherosclerosis in a hyperglycaemic mouse model. Antioxid. Redox. Signal. 11, 2289–2298 - PubMed
-
- Kurokawa M., Hideshima M., Ishii Y., Kyuwa S., Yoshikawa Y. (2009) Aortic ER stress in streptozotocin-induced diabetes mellitus in APA hamsters. Exp. Anim. 58, 113–121 - PubMed
-
- Qi W., Mu J., Luo Z. F., Zeng W., Guo Y. H., Pang Q., Ye Z. L., Liu L., Yuan F. H., Feng B. (2011) Attenuation of diabetic nephropathy in diabetes rats induced by streptozotocin by regulating the endoplasmic reticulum stress inflammatory response. Metabolism 60, 594–603 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

