Always cleave up your mess: targeting collagen degradation to treat tissue fibrosis
- PMID: 23564511
- PMCID: PMC3680761
- DOI: 10.1152/ajplung.00418.2012
Always cleave up your mess: targeting collagen degradation to treat tissue fibrosis
Abstract
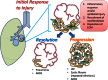
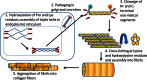
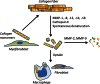
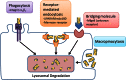
Pulmonary fibrosis is a vexing clinical problem with no proven therapeutic options. In the normal lung there is continuous collagen synthesis and collagen degradation, and these two processes are precisely balanced to maintain normal tissue architecture. With lung injury there is an increase in the rate of both collagen production and collagen degradation. The increase in collagen degradation is critical in preventing the formation of permanent scar tissue each time the lung is exposed to injury. In pulmonary fibrosis, collagen degradation does not keep pace with collagen production, resulting in extracellular accumulation of fibrillar collagen. Collagen degradation occurs through both extracellular and intracellular pathways. The extracellular pathway involves cleavage of collagen fibrils by proteolytic enzyme including the metalloproteinases. The less-well-described intracellular pathway involves binding and uptake of collagen fragments by fibroblasts and macrophages for lysosomal degradation. The relationship between these two pathways and their relevance to the development of fibrosis is complex. Fibrosis in the lung, liver, and skin has been associated with an impaired degradative environment. Much of the current scientific effort in fibrosis is focused on understanding the pathways that regulate increased collagen production. However, recent reports suggest an important role for collagen turnover and degradation in regulating the severity of tissue fibrosis. The objective of this review is to evaluate the roles of the extracellular and intracellular collagen degradation pathways in the development of fibrosis and to examine whether pulmonary fibrosis can be viewed as a disease of impaired matrix degradation rather than a disease of increased matrix production.
Keywords: UIP; collagen degradation; extracellular matrix; matrix metalloproteinases; pulmonary fibrosis.
Figures
References
-
- Armstrong L, Thickett DR, Mansell JP, Ionescu M, Hoyle E, Billinghurst RC, Poole AR, Millar AB. Changes in collagen turnover in early acute respiratory distress syndrome. Am J Respir Crit Care Med 160: 1910–1915, 1999 - PubMed
-
- Arora PD, Manolson MF, Downey GP, Sodek J, McCulloch CA. A novel model system for characterization of phagosomal maturation, acidification, and intracellular collagen degradation in fibroblasts. J Biol Chem 275: 35432–35441, 2000 - PubMed
-
- Atabai K, Jame S, Azhar N, Kuo A, Lam M, McKleroy W, Dehart G, Rahman S, Xia DD, Melton AC, Wolters P, Emson CL, Turner SM, Werb Z, Sheppard D. Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages. J Clin Invest 119: 3713–3722, 2009 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

