The role of dendritic inhibition in shaping the plasticity of excitatory synapses
- PMID: 23565076
- PMCID: PMC3615258
- DOI: 10.3389/fncir.2012.00118
The role of dendritic inhibition in shaping the plasticity of excitatory synapses
Abstract
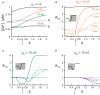
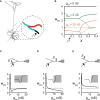
Using computational tools we explored the impact of local synaptic inhibition on the plasticity of excitatory synapses in dendrites. The latter critically depends on the intracellular concentration of calcium, which in turn, depends on membrane potential and thus on inhibitory activity in particular dendritic compartments. We systematically characterized the dependence of excitatory synaptic plasticity on dendritic morphology, loci and strength, as well as on the spatial distribution of inhibitory synapses and on the level of excitatory activity. Plasticity of excitatory synapses may attain three states: "protected" (unchanged), potentiated (long-term potentiation; LTP), or depressed (long-term depression; LTD). The transition between these three plasticity states could be finely tuned by synaptic inhibition with high spatial resolution. Strategic placement of inhibition could give rise to the co-existence of all three states over short dendritic branches. We compared the plasticity effect of the innervation patterns typical of different inhibitory subclasses-Chandelier, Basket, Martinotti, and Double Bouquet-in a detailed model of a layer 5 pyramidal cell. Our study suggests that dendritic inhibition plays a key role in shaping and fine-tuning excitatory synaptic plasticity in dendrites.
Keywords: compartmental model; dendritic cable; dendritic calcium; dendritic inhibition; synaptic plasticity.
Figures




References
-
- Artola A., Singer W. (1993). Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 16, 480–487 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

