Conformational and functional effects induced by D- and L-amino acid epimerization on a single gene encoded peptide from the skin secretion of Hypsiboas punctatus
- PMID: 23565145
- PMCID: PMC3614549
- DOI: 10.1371/journal.pone.0059255
Conformational and functional effects induced by D- and L-amino acid epimerization on a single gene encoded peptide from the skin secretion of Hypsiboas punctatus
Abstract
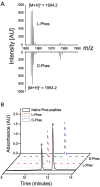
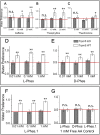
Skin secretion of Hypsiboas punctatus is the source of a complex mixture of bioactive compounds where peptides and small proteins prevail, similarly to many other amphibians. Among dozens of molecules isolated from H. punctatus in a proteomic based approach, we report here the structural and functional studies of a novel peptide named Phenylseptin (FFFDTLKNLAGKVIGALT-NH2) that was purified as two naturally occurring D- and L-Phes configurations. The amino acid epimerization and C-terminal amidation for both molecules were confirmed by a combination of techniques including reverse-phase UFLC, ion mobility mass spectrometry, high resolution MS/MS experiments, Edman degradation, cDNA sequencing and solid-phase peptide synthesis. RMSD analysis of the twenty lowest-energy (1)H NMR structures of each peptide revealed a major 90° difference between the two backbones at the first four N-terminal residues and substantial orientation changes of their respective side chains. These structural divergences were considered to be the primary cause of the in vitro quantitative differences in antimicrobial activities between the two molecules. Finally, both molecules elicited equally aversive reactions in mice when delivered orally, an effect that depended entirely on peripheral gustatory pathways.
Conflict of interest statement
Figures



Similar articles
-
Structural and functional characterization of two genetically related meucin peptides highlights evolutionary divergence and convergence in antimicrobial peptides.FASEB J. 2009 Apr;23(4):1230-45. doi: 10.1096/fj.08-122317. Epub 2008 Dec 16. FASEB J. 2009. PMID: 19088182
-
Antimicrobial peptides and alytesin are co-secreted from the venom of the Midwife toad, Alytes maurus (Alytidae, Anura): implications for the evolution of frog skin defensive secretions.Toxicon. 2012 Nov;60(6):967-81. doi: 10.1016/j.toxicon.2012.06.015. Epub 2012 Jul 16. Toxicon. 2012. PMID: 22800568
-
Skin secretion peptides: the molecular facet of the deimatic behavior of the four-eyed frog, Physalaemus nattereri (Anura, Leptodactylidae).Rapid Commun Mass Spectrom. 2015 Nov 15;29(21):2061-8. doi: 10.1002/rcm.7313. Rapid Commun Mass Spectrom. 2015. PMID: 26443407
-
A lesson from Bombinins H, mildly cationic diastereomeric antimicrobial peptides from Bombina skin.Curr Protein Pept Sci. 2013 Dec;14(8):734-43. doi: 10.2174/138920371408131227171817. Curr Protein Pept Sci. 2013. PMID: 24384035 Review.
-
The world of beta- and gamma-peptides comprised of homologated proteinogenic amino acids and other components.Chem Biodivers. 2004 Aug;1(8):1111-239. doi: 10.1002/cbdv.200490087. Chem Biodivers. 2004. PMID: 17191902 Review.
Cited by
-
Linear and Differential Ion Mobility Separations of Middle-Down Proteoforms.Anal Chem. 2018 Feb 20;90(4):2918-2925. doi: 10.1021/acs.analchem.7b05224. Epub 2018 Feb 6. Anal Chem. 2018. PMID: 29359922 Free PMC article.
-
Cleavage of Peptides from Amphibian Skin Revealed by Combining Analysis of Gland Secretion and in Situ MALDI Imaging Mass Spectrometry.ACS Omega. 2018 May 31;3(5):5426-5434. doi: 10.1021/acsomega.7b02029. Epub 2018 May 21. ACS Omega. 2018. PMID: 30023919 Free PMC article.
-
Enhanced ion mobility resolution of Abeta isomers from human brain using high-resolution demultiplexing software.Anal Bioanal Chem. 2022 Jul;414(18):5683-5693. doi: 10.1007/s00216-022-04055-x. Epub 2022 Apr 15. Anal Bioanal Chem. 2022. PMID: 35426495
-
Site-specific characterization of (D)-amino acid containing peptide epimers by ion mobility spectrometry.Anal Chem. 2014 Mar 18;86(6):2972-81. doi: 10.1021/ac4033824. Epub 2014 Jan 3. Anal Chem. 2014. PMID: 24328107 Free PMC article.
-
Quantification of N-terminal amyloid-β isoforms reveals isomers are the most abundant form of the amyloid-β peptide in sporadic Alzheimer's disease.Brain Commun. 2021 Mar 9;3(2):fcab028. doi: 10.1093/braincomms/fcab028. eCollection 2021. Brain Commun. 2021. PMID: 33928245 Free PMC article.
References
-
- Poyurovsky M, Weizman R, Weizman A (2011) Mirtazapine - a multifunctional drug: low dose for akathisia. CNS Spectr 16: 63. - PubMed
-
- Trial TIS (1997) A randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet 349: 1569–1581. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

