The anti-scar effects of basic fibroblast growth factor on the wound repair in vitro and in vivo
- PMID: 23565178
- PMCID: PMC3615060
- DOI: 10.1371/journal.pone.0059966
The anti-scar effects of basic fibroblast growth factor on the wound repair in vitro and in vivo
Abstract
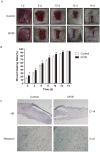
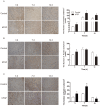
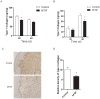
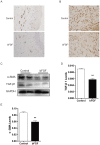
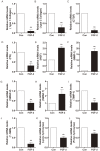
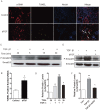
Hypertrophic scars (HTS) and keloids are challenging problems. Their pathogenesis results from an overproduction of fibroblasts and excessive deposition of collagen. Studies suggest a possible anti-scarring effect of basic fibroblast growth factor (bFGF) during wound healing, but the precise mechanisms of bFGF are still unclear. In view of this, we investigated the therapeutic effects of bFGF on HTS animal model as well as human scar fibroblasts (HSF) model. We show that bFGF promoted wound healing and reduced the area of flattened non-pathological scars in rat skin wounds and HTS in the rabbit ear. We provide evidence of a new therapeutic strategy: bFGF administration for the treatment of HTS. The scar elevation index (SEI) and epidermal thickness index (ETI) was also significantly reduced. Histological reveal that bFGF exhibited significant amelioration of the collagen tissue. bFGF regulated extracellular matrix (ECM) synthesis and degradation via interference in the collagen distribution, the α-smooth muscle actin (α-SMA) and transforming growth factor-1 (TGF-β1) expression. In addition, bFGF reduced scarring and promoted wound healing by inhibiting TGFβ1/SMAD-dependent pathway. The levels of fibronectin (FN), tissue inhibitor of metalloproteinase-1 (TIMP-1) collagen I, and collagen III were evidently decreased, and matrix metalloproteinase-1 (MMP-1) and apoptosis cells were markedly increased. These results suggest that bFGF possesses favorable therapeutic effects on hypertrophic scars in vitro and in vivo, which may be an effective cure for human hypertrophic scars.
Conflict of interest statement
Figures

Similar articles
-
Wound treatment with curcumin prevents hypertrophic scarring and promotes remodeling by inhibiting fibroblast activation and regulating collagen deposition.Arch Dermatol Res. 2025 May 17;317(1):767. doi: 10.1007/s00403-025-04271-2. Arch Dermatol Res. 2025. PMID: 40381057
-
Normal skin and hypertrophic scar fibroblasts differentially regulate collagen and fibronectin expression as well as mitochondrial membrane potential in response to basic fibroblast growth factor.Braz J Med Biol Res. 2011 May;44(5):402-10. doi: 10.1590/S0100-879X2011007500041. Epub 2011 Apr 1. Braz J Med Biol Res. 2011. PMID: 21445528
-
Basic fibroblast growth factor (bFGF) alleviates the scar of the rabbit ear model in wound healing.Wound Repair Regen. 2008 Jul-Aug;16(4):576-81. doi: 10.1111/j.1524-475X.2008.00405.x. Wound Repair Regen. 2008. PMID: 18638277
-
Dermal Fibroblast Heterogeneity and Its Contribution to the Skin Repair and Regeneration.Adv Wound Care (New Rochelle). 2022 Feb;11(2):87-107. doi: 10.1089/wound.2020.1287. Epub 2021 Mar 23. Adv Wound Care (New Rochelle). 2022. PMID: 33607934 Review.
-
Inflammation in Wound Healing and Pathological Scarring.Adv Wound Care (New Rochelle). 2023 May;12(5):288-300. doi: 10.1089/wound.2021.0161. Adv Wound Care (New Rochelle). 2023. PMID: 36541356 Review.
Cited by
-
Alteration of skin properties with autologous dermal fibroblasts.Int J Mol Sci. 2014 May 13;15(5):8407-27. doi: 10.3390/ijms15058407. Int J Mol Sci. 2014. PMID: 24828202 Free PMC article. Review.
-
Fibroblast growth factor 2 accelerates the epithelial-mesenchymal transition in keratinocytes during wound healing process.Sci Rep. 2020 Oct 29;10(1):18545. doi: 10.1038/s41598-020-75584-7. Sci Rep. 2020. PMID: 33122782 Free PMC article.
-
The Influence of Sulfation Degree of Glycosaminoglycan-Functionalized 3D Collagen I Networks on Cytokine Profiles of In Vitro Macrophage-Fibroblast Cocultures.Gels. 2024 Jul 9;10(7):450. doi: 10.3390/gels10070450. Gels. 2024. PMID: 39057473 Free PMC article.
-
Platelet-rich plasma: a comparative and economical therapy for wound healing and tissue regeneration.Cell Tissue Bank. 2023 Jun;24(2):285-306. doi: 10.1007/s10561-022-10039-z. Epub 2022 Oct 12. Cell Tissue Bank. 2023. PMID: 36222966 Free PMC article. Review.
-
Enhanced effect of fibroblast growth factor-2-containing dalteparin/protamine nanoparticles on hair growth.Clin Cosmet Investig Dermatol. 2016 May 17;9:127-34. doi: 10.2147/CCID.S108187. eCollection 2016. Clin Cosmet Investig Dermatol. 2016. PMID: 27274299 Free PMC article.
References
-
- Grieb G, Steffens G, Pallua N, Bernhagen J, Bucala R (2011) Circulating fibrocytes–biology and mechanisms in wound healing and scar formation. Int Rev Cell Mol Biol 291: 1–19. - PubMed
-
- Wang W, Lin SQ, Xiao YC, Huang YD, Tan Y, et al. (2008) Acceleration of diabetic wound healing with chitosan-crosslinked collagen sponge containing recombinant human acidic fibroblast growth factor in healing-impaired STZ diabetic rats. Life Sciences 82: 190–204. - PubMed
-
- Xiao J, Lv YX, Lin SQ, Jin LT, Zhang Y, et al. (2010) Cardiac Protection by Basic Fibroblast Growth Factor from Ischemia/Reperfusion-Induced Injury in Diabetic Rats. Biological & Pharmaceutical Bulletin 33: 444–449. - PubMed
-
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M (2008) Growth factors and cytokines in wound healing. Wound Repair Regen 16: 585–601. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

