SMA-MAP: a plasma protein panel for spinal muscular atrophy
- PMID: 23565191
- PMCID: PMC3615018
- DOI: 10.1371/journal.pone.0060113
SMA-MAP: a plasma protein panel for spinal muscular atrophy
Abstract
Objectives: Spinal Muscular Atrophy (SMA) presents challenges in (i) monitoring disease activity and predicting progression, (ii) designing trials that allow rapid assessment of candidate therapies, and (iii) understanding molecular causes and consequences of the disease. Validated biomarkers of SMA motor and non-motor function would offer utility in addressing these challenges. Our objectives were (i) to discover additional markers from the Biomarkers for SMA (BforSMA) study using an immunoassay platform, and (ii) to validate the putative biomarkers in an independent cohort of SMA patients collected from a multi-site natural history study (NHS).
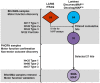
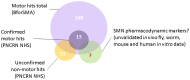
Methods: BforSMA study plasma samples (N = 129) were analyzed by immunoassay to identify new analytes correlating to SMA motor function. These immunoassays included the strongest candidate biomarkers identified previously by chromatography. We selected 35 biomarkers to validate in an independent cohort SMA type 1, 2, and 3 samples (N = 158) from an SMA NHS. The putative biomarkers were tested for association to multiple motor scales and to pulmonary function, neurophysiology, strength, and quality of life measures. We implemented a Tobit model to predict SMA motor function scores.
Results: 12 of the 35 putative SMA biomarkers were significantly associated (p<0.05) with motor function, with a 13(th) analyte being nearly significant. Several other analytes associated with non-motor SMA outcome measures. From these 35 biomarkers, 27 analytes were selected for inclusion in a commercial panel (SMA-MAP) for association with motor and other functional measures.
Conclusions: Discovery and validation using independent cohorts yielded a set of SMA biomarkers significantly associated with motor function and other measures of SMA disease activity. A commercial SMA-MAP biomarker panel was generated for further testing in other SMA collections and interventional trials. Future work includes evaluating the panel in other neuromuscular diseases, for pharmacodynamic responsiveness to experimental SMA therapies, and for predicting functional changes over time in SMA patients.
Conflict of interest statement
Figures


References
-
- Van Meerbeke JP, Sumner CJ (2011) Progress and promise: the current status of spinal muscular atrophy therapeutics. Discov Med 12: 291–305. - PubMed
-
- Mercuri E, Bertini E, Messina S, Pelliccioni M, D’Amico A, et al. (2004) Pilot trial of phenylbutyrate in spinal muscular atrophy. Neuromuscul Disord 14: 130–135. - PubMed
-
- Pane M, Staccioli S, Messina S, D’Amico A, Pelliccioni M, et al. (2008) Daily salbutamol in young patients with SMA type II. Neuromuscul Disord 18: 536–540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

