From patient-specific mathematical neuro-oncology to precision medicine
- PMID: 23565501
- PMCID: PMC3613895
- DOI: 10.3389/fonc.2013.00062
From patient-specific mathematical neuro-oncology to precision medicine
Abstract
Gliomas are notoriously aggressive, malignant brain tumors that have variable response to treatment. These patients often have poor prognosis, informed primarily by histopathology. Mathematical neuro-oncology (MNO) is a young and burgeoning field that leverages mathematical models to predict and quantify response to therapies. These mathematical models can form the basis of modern "precision medicine" approaches to tailor therapy in a patient-specific manner. Patient-specific models (PSMs) can be used to overcome imaging limitations, improve prognostic predictions, stratify patients, and assess treatment response in silico. The information gleaned from such models can aid in the construction and efficacy of clinical trials and treatment protocols, accelerating the pace of clinical research in the war on cancer. This review focuses on the growing translation of PSM to clinical neuro-oncology. It will also provide a forward-looking view on a new era of patient-specific MNO.
Keywords: clinical modeling; glioma; individualized health care; mathematical modeling; patient-specific; personalized medicine.
Figures
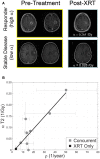
Similar articles
-
Patient-specific mathematical neuro-oncology: using a simple proliferation and invasion tumor model to inform clinical practice.Bull Math Biol. 2015 May;77(5):846-56. doi: 10.1007/s11538-015-0067-7. Epub 2015 Mar 21. Bull Math Biol. 2015. PMID: 25795318 Free PMC article. Review.
-
Personalized and translational approach for malignant brain tumors in the era of precision medicine: the strategic contribution of an experienced neurosurgery laboratory in a modern neurosurgery and neuro-oncology department.J Neurol Sci. 2020 Oct 15;417:117083. doi: 10.1016/j.jns.2020.117083. Epub 2020 Aug 6. J Neurol Sci. 2020. PMID: 32784071 Review.
-
In Silico Mathematical Modelling for Glioblastoma: A Critical Review and a Patient-Specific Case.J Clin Med. 2021 May 17;10(10):2169. doi: 10.3390/jcm10102169. J Clin Med. 2021. PMID: 34067871 Free PMC article. Review.
-
Incorporation of prognostic and predictive factors into glioma clinical trials.Curr Oncol Rep. 2013 Feb;15(1):56-63. doi: 10.1007/s11912-012-0279-z. Curr Oncol Rep. 2013. PMID: 23125011 Free PMC article. Review.
-
Personalized care in neuro-oncology coming of age: why we need MGMT and 1p/19q testing for malignant glioma patients in clinical practice.Neuro Oncol. 2012 Sep;14 Suppl 4(Suppl 4):iv100-8. doi: 10.1093/neuonc/nos206. Neuro Oncol. 2012. PMID: 23095825 Free PMC article. Review.
Cited by
-
Opportunities for improving brain cancer treatment outcomes through imaging-based mathematical modeling of the delivery of radiotherapy and immunotherapy.Adv Drug Deliv Rev. 2022 Aug;187:114367. doi: 10.1016/j.addr.2022.114367. Epub 2022 May 30. Adv Drug Deliv Rev. 2022. PMID: 35654212 Free PMC article. Review.
-
Simulating cancer: computational models in oncology.Front Oncol. 2013 Sep 13;3:233. doi: 10.3389/fonc.2013.00233. eCollection 2013. Front Oncol. 2013. PMID: 24062986 Free PMC article. No abstract available.
-
Towards personalized computational oncology: from spatial models of tumour spheroids, to organoids, to tissues.J R Soc Interface. 2018 Jan;15(138):20170703. doi: 10.1098/rsif.2017.0703. J R Soc Interface. 2018. PMID: 29367239 Free PMC article. Review.
-
Clinical implications of in silico mathematical modeling for glioblastoma: a critical review.J Neurooncol. 2018 Jan;136(1):1-11. doi: 10.1007/s11060-017-2650-2. Epub 2017 Oct 28. J Neurooncol. 2018. PMID: 29081039 Review.
-
MRI-based digital twins to improve treatment response of breast cancer by optimizing neoadjuvant chemotherapy regimens.NPJ Digit Med. 2025 Apr 7;8(1):195. doi: 10.1038/s41746-025-01579-1. NPJ Digit Med. 2025. PMID: 40195521 Free PMC article.
References
-
- Baldock A. L., Anh S., Rockne R., Neal M., Clark-Swanson K., Sterin G., et al. (2012a). Patient-specific invasiveness metric predicts benefit of resection in human gliomas. Neuro-oncology 14, 131–131
-
- Baldock A. L., Yagle K., Anh S., Born D., Swanson P., Rockne R., et al. (2012b). Invasion and proliferation kinetics predict IDH-1 mutation in contrast-enhancing gliomas. Neuro-oncology 14, 131–131
-
- Bohman L. E., Swanson K. R., Moore J. L., Rockne R., Mandigo C., Hankinson T., et al. (2010). Magnetic resonance imaging characteristics of glioblastoma multiforme: implications for understanding glioma ontogeny. Neurosurgery 67, 1319–1327; discussion 1318–1327.10.1227/NEU.0b013e3181f556ab - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

