Developing ligands for palladium(II)-catalyzed C-H functionalization: intimate dialogue between ligand and substrate
- PMID: 23565982
- PMCID: PMC3779523
- DOI: 10.1021/jo400159y
Developing ligands for palladium(II)-catalyzed C-H functionalization: intimate dialogue between ligand and substrate
Abstract
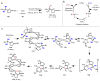
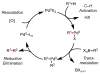
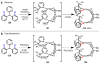
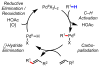
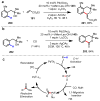
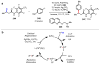
Homogeneous transition-metal-catalyzed reactions are indispensable to all facets of modern chemical synthesis. It is thus difficult to imagine that for much of the early 20th century, the reactivity and selectivity of all known homogeneous metal catalysts paled in comparison to their heterogeneous and biological counterparts. In the intervening decades, advances in ligand design bridged this divide, such that today some of the most demanding bond-forming events are mediated by ligand-supported homogeneous metal species. While ligand design has propelled many areas of homogeneous catalysis, in the field of Pd(II)-catalyzed C-H functionalization, suitable ligand scaffolds are lacking, which has hampered the development of broadly practical transformations based on C-H functionalization logic. In this Perspective, we offer an account of our research employing three ligand scaffolds, mono-N-protected amino acids, 2,6-disubstituted pyridines, and 2,2'-bipyridines, to address challenges posed by several synthetically versatile substrate classes. Drawing on this work, we discuss principles of ligand design, such as the need to match a ligand to a particular substrate class, and how ligand traits such as tunability and modularity can be advantageous in reaction discovery.
Figures




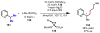


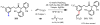


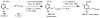
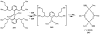

References
-
- Berrisford DJ, Bolm C, Sharpless KB. Angew Chem Int Ed. 1995;34:1059–1070.
-
-
As a point of clarification, ‘ligand’ can refer to any molecule that is coordinated to a metal catalyst (anionic donor, X-type; or neutral donor, L-type). Throughout the text we generally focus on ligands that possesses an element of design (i.e., a molecule that was independently synthesized for the express purpose of coordinating it to the metal to influence the reactivity), rather than common counterions (OAc, OTf, Cl, etc.) or solvent molecules (MeCN, THF, etc.), but those too can have a dramatic impact on the reactivity and/or selectivity of catalytic transformations.
-
-
- Katsuki T, Sharpless KB. J Am Chem Soc. 1980;102:5974–5976.
-
- Gao Y, Hanson RM, Klunder JM, Ko SY, Masamune H, Sharpless KB. J Am Chem Soc. 1987;109:5765–5780.
-
- Dang TP, Kagan HB. J Chem Soc D. 1971:481.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

