Lateral inhibition in visual cortex of migraine patients between attacks
- PMID: 23565983
- PMCID: PMC3620512
- DOI: 10.1186/1129-2377-14-20
Lateral inhibition in visual cortex of migraine patients between attacks
Abstract
Background: The interictal deficit of habituation to repetitive visual stimuli in migraine patients could be due to deficient intracortical inhibition and/or to low cortical pre-activation levels. Which of these abnormalities contributes more to the habituation deficit cannot be determined with the common methods used to record transient visual responses.We investigated lateral inhibition in the visual cortex during the migraine cycle and in healthy subjects by using differential temporal modulations of radial windmill-dartboard (WD) or partial-windmill (PW) visual patterns.
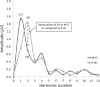
Methods: Transient (TR-VEP) and steady-state visual-evoked potentials (SS-VEP) were recorded in 65 migraine patients (21 without and 22 with aura between attacks; 22 patients during an attack) and in 21 healthy volunteers (HV). Three stimulations were used in each subject: classic checkerboard pattern (contrast-reversion 3.1 Hz), WD and PW (contrast-reversion ~4 Hz). For each randomly presented stimulation protocol, 600 sweeps were acquired and off-line partitioned in 6 blocks of 100. Fourier analysis allowed data to extract in SS-VEP the fundamental (1H) and the second harmonic (2H) components that reflect respectively short-(WD) and long- range lateral inhibition (attenuation of 2H in WD compared to PW).
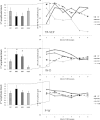
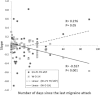
Results: Compared to HV, migraineurs recorded interictally had significantly less habituation of the N1-P1 TR-VEP component over subsequent blocks and they tended to have a smaller 1st block amplitude. 1H amplitude in the 1st block of WD SS-VEP was significantly greater than in HV and habituated in successive blocks, contrasting with an amplitude increase in HV. Both the interictal TR-VEP and SS-VEP abnormalities normalized during an attack. There was no significant between group difference in the PW 2H amplitude and its attenuation. When data of HV and migraine patients were combined, the habituation slope of WD-VEP 1H was negatively correlated with that of TR-VEP N1-P1 and with number of days since the last migraine attack.
Conclusion: These results are in favour of a migraine cycle-dependent imbalance between excitation and inhibition in the visual cortex. We hypothesize that an interictal hypoactivity of monaminergic pathways may cause a functional disconnection of the thalamus in migraine leading to an abnormal intracortical short-range lateral inhibition that could contribute to the habituation deficit observed during stimulus repetition.
Figures



Similar articles
-
Effects of light deprivation on visual evoked potentials in migraine without aura.BMC Neurol. 2011 Jul 27;11:91. doi: 10.1186/1471-2377-11-91. BMC Neurol. 2011. PMID: 21794160 Free PMC article.
-
Effects of repetitive transcranial magnetic stimulation on visual evoked potentials in migraine.Brain. 2002 Apr;125(Pt 4):912-22. doi: 10.1093/brain/awf081. Brain. 2002. PMID: 11912123
-
Familial history of migraine influences habituation of visual evoked potentials.Cephalalgia. 2017 Oct;37(11):1082-1087. doi: 10.1177/0333102416673207. Epub 2016 Nov 12. Cephalalgia. 2017. PMID: 27821640
-
Highlights in migraine electrophysiology: are controversies just reflecting disease heterogeneity?Curr Opin Neurol. 2016 Jun;29(3):320-30. doi: 10.1097/WCO.0000000000000335. Curr Opin Neurol. 2016. PMID: 27120104 Review.
-
Electrophysiological studies in migraine: a comprehensive review of their interest and limitations.Cephalalgia. 2003;23 Suppl 1:13-31. doi: 10.1046/j.1468-2982.2003.00571.x. Cephalalgia. 2003. PMID: 12699456 Review.
Cited by
-
Preliminary evidence of reduced brain network activation in patients with post-traumatic migraine following concussion.Brain Imaging Behav. 2016 Jun;10(2):594-603. doi: 10.1007/s11682-015-9412-6. Brain Imaging Behav. 2016. PMID: 26091725 Free PMC article.
-
Habituation and sensitization in primary headaches.J Headache Pain. 2013 Jul 30;14(1):65. doi: 10.1186/1129-2377-14-65. J Headache Pain. 2013. PMID: 23899115 Free PMC article. Review.
-
Afterimage duration differs for migraine with or without aura.Headache. 2025 May;65(5):756-763. doi: 10.1111/head.14934. Epub 2025 Mar 28. Headache. 2025. PMID: 40152333 Free PMC article.
-
Pain-Related Brain Connectivity Changes in Migraine: A Narrative Review and Proof of Concept about Possible Novel Treatments Interference.Brain Sci. 2021 Feb 13;11(2):234. doi: 10.3390/brainsci11020234. Brain Sci. 2021. PMID: 33668449 Free PMC article. Review.
-
Abnormalities in cortical pattern of coherence in migraine detected using ultra high-density EEG.Brain Commun. 2021 Apr 2;3(2):fcab061. doi: 10.1093/braincomms/fcab061. eCollection 2021. Brain Commun. 2021. PMID: 34258580 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

