Modeling vascularized bone regeneration within a porous biodegradable CaP scaffold loaded with growth factors
- PMID: 23566802
- PMCID: PMC3770300
- DOI: 10.1016/j.biomaterials.2013.03.015
Modeling vascularized bone regeneration within a porous biodegradable CaP scaffold loaded with growth factors
Abstract
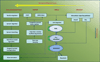
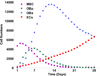
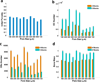
Osteogenetic microenvironment is a complex constitution in which extracellular matrix (ECM) molecules, stem cells and growth factors each interact to direct the coordinate regulation of bone tissue development. Importantly, angiogenesis improvement and revascularization are critical for osteogenesis during bone tissue regeneration processes. In this study, we developed a three-dimensional (3D) multi-scale system model to study cell response to growth factors released from a 3D biodegradable porous calcium phosphate (CaP) scaffold. Our model reconstructed the 3D bone regeneration system and examined the effects of pore size and porosity on bone formation and angiogenesis. The results suggested that scaffold porosity played a more dominant role in affecting bone formation and angiogenesis compared with pore size, while the pore size could be controlled to tailor the growth factor release rate and release fraction. Furthermore, a combination of gradient VEGF with BMP2 and Wnt released from the multi-layer scaffold promoted angiogenesis and bone formation more readily than single growth factors. These results demonstrated that the developed model can be potentially applied to predict vascularized bone regeneration with specific scaffold and growth factors.
Published by Elsevier Ltd.
Figures




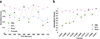
References
-
- Petite H, Viateau V, Bensaid W, Meunier A, De Pollak C, Bourguignon M, et al. Tissue-engineered bone regeneration. Nature Biotechnol. 2000;18:959–963. - PubMed
-
- Dragoo JL, Lieberman JR, Lee RS, Deugarte DA, Lee Y, Zuk PA, et al. Tissue-engineered bone from BMP-2--transduced stem cells derived from human fat. Plast Reconstr Surg. 2005;115:1665–1673. - PubMed
-
- Trautvetter W, Kaps C, Schmelzeisen R, Sauerbier S, Sittinger M. Tissue-engineered polymer-based periosteal bone grafts for maxillary sinus augmentation: five-year clinical results. J Oral Maxillofac Surg. 2011;69:2753–2762. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 LM010185/LM/NLM NIH HHS/United States
- R01LM010185-03/LM/NLM NIH HHS/United States
- R01DE021468/DE/NIDCR NIH HHS/United States
- R01 DE022676/DE/NIDCR NIH HHS/United States
- U01 CA166886-01/CA/NCI NIH HHS/United States
- U01HL111560-01/HL/NHLBI NIH HHS/United States
- U01 CA166886/CA/NCI NIH HHS/United States
- 1R01DE022676-01/DE/NIDCR NIH HHS/United States
- U01 HL111560/HL/NHLBI NIH HHS/United States
- R01AR057837/AR/NIAMS NIH HHS/United States
- R01 DE021468/DE/NIDCR NIH HHS/United States
- R01 AR057837/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

