A Conus regularis conotoxin with a novel eight-cysteine framework inhibits CaV2.2 channels and displays an anti-nociceptive activity
- PMID: 23567319
- PMCID: PMC3705398
- DOI: 10.3390/md11041188
A Conus regularis conotoxin with a novel eight-cysteine framework inhibits CaV2.2 channels and displays an anti-nociceptive activity
Abstract
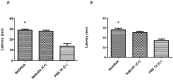
A novel peptide, RsXXIVA, was isolated from the venom duct of Conus regularis, a worm-hunting species collected in the Sea of Cortez, México. Its primary structure was determined by mass spectrometry and confirmed by automated Edman degradation. This conotoxin contains 40 amino acids and exhibits a novel arrangement of eight cysteine residues (C-C-C-C-CC-CC). Surprisingly, two loops of the novel peptide are highly identical to the amino acids sequence of ω-MVIIA. The total length and disulfide pairing of both peptides are quite different, although the two most important residues for the described function of ω-MVIIA (Lys2 and Tyr13) are also present in the peptide reported here. Electrophysiological analysis using superior cervical ganglion (SCG) neurons indicates that RsXXIVA inhibits CaV2.2 channel current in a dose-dependent manner with an EC50 of 2.8 μM, whose effect is partially reversed after washing. Furthermore, RsXXIVA was tested in hot-plate assays to measure the potential anti-nociceptive effect to an acute thermal stimulus, showing an analgesic effect in acute thermal pain at 30 and 45 min post-injection. Also, the toxin shows an anti-nociceptive effect in a formalin chronic pain test. However, the low affinity for CaV2.2 suggests that the primary target of the peptide could be different from that of ω-MVIIA.
Figures

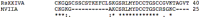



Similar articles
-
A novel α-conopeptide Eu1.6 inhibits N-type (CaV2.2) calcium channels and exhibits potent analgesic activity.Sci Rep. 2018 Jan 17;8(1):1004. doi: 10.1038/s41598-017-18479-4. Sci Rep. 2018. PMID: 29343689 Free PMC article.
-
The synthesis of SO-3, a conopeptide with high analgesic activity derived from Conus striatus.J Nat Prod. 2003 Sep;66(9):1276-9. doi: 10.1021/np030099y. J Nat Prod. 2003. PMID: 14510617
-
Novel analgesic ω-conotoxins from the vermivorous cone snail Conus moncuri provide new insights into the evolution of conopeptides.Sci Rep. 2018 Sep 7;8(1):13397. doi: 10.1038/s41598-018-31245-4. Sci Rep. 2018. PMID: 30194442 Free PMC article.
-
Analgesic conotoxins: block and G protein-coupled receptor modulation of N-type (Ca(V) 2.2) calcium channels.Br J Pharmacol. 2012 May;166(2):486-500. doi: 10.1111/j.1476-5381.2011.01781.x. Br J Pharmacol. 2012. PMID: 22091786 Free PMC article. Review.
-
Bioactive Mimetics of Conotoxins and other Venom Peptides.Toxins (Basel). 2015 Oct 16;7(10):4175-98. doi: 10.3390/toxins7104175. Toxins (Basel). 2015. PMID: 26501323 Free PMC article. Review.
Cited by
-
Conotoxin gene superfamilies.Mar Drugs. 2014 Dec 17;12(12):6058-101. doi: 10.3390/md12126058. Mar Drugs. 2014. PMID: 25522317 Free PMC article. Review.
-
A Transcriptomic Survey of Ion Channel-Based Conotoxins in the Chinese Tubular Cone Snail (Conus betulinus).Mar Drugs. 2017 Jul 18;15(7):228. doi: 10.3390/md15070228. Mar Drugs. 2017. PMID: 28718820 Free PMC article.
-
Discovery Methodology of Novel Conotoxins from Conus Species.Mar Drugs. 2018 Oct 30;16(11):417. doi: 10.3390/md16110417. Mar Drugs. 2018. PMID: 30380764 Free PMC article. Review.
-
Lybatides from Lycium barbarum Contain An Unusual Cystine-stapled Helical Peptide Scaffold.Sci Rep. 2017 Jul 12;7(1):5194. doi: 10.1038/s41598-017-05037-1. Sci Rep. 2017. PMID: 28701689 Free PMC article.
-
High-throughput identification of novel conotoxins from the Chinese tubular cone snail (Conus betulinus) by multi-transcriptome sequencing.Gigascience. 2016 Apr 14;5:17. doi: 10.1186/s13742-016-0122-9. eCollection 2016. Gigascience. 2016. PMID: 27087938 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

