Lack of Cyp1b1 promotes the proliferative and migratory phenotype of perivascular supporting cells
- PMID: 23568032
- PMCID: PMC3791926
- DOI: 10.1038/labinvest.2013.55
Lack of Cyp1b1 promotes the proliferative and migratory phenotype of perivascular supporting cells
Abstract
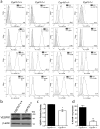
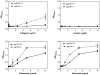
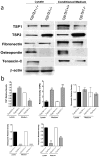
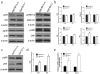
Perivascular supporting cells, including pericytes and smooth muscle cells (PC/SMC), have an integral role during angiogenesis and control vascular remodeling, maturation, and stabilization of neoteric vessels. We recently showed that a Cyp1B1 deficiency in mice results in the attenuation of angiogenesis in vivo and the pro-angiogenic activity of endothelial cells in vitro. However, the contribution of PC/SMC, and more specifically the cell autonomous effects of Cyp1B1 in these processes, needs further investigation. Here we demonstrate that PC constitutively expressed Cyp1B1, and that a deficiency in Cyp1B1 was associated with enhanced proliferation, and decreased apoptosis. Mechanistically, the lack of Cyp1B1 was associated with increased oxidative stress and sustained NF-κB activation, which was reversed by the antioxidant N-acetylcysteine. These changes were also concomitant with alterations in PC migration, adhesion, and expression of various extracellular matrix proteins, including thrombospondin-2. Cyp1B1-deficient PC also expressed decreased levels of vascular endothelial growth factor. Together, our results suggest an important role for Cyp1B1 expression in the regulation of PC proliferation, migration, and survival through modulation of the intracellular oxidative state and NF-κB expression and/or activity. Thus, a lack of Cyp1B1 in PC may have a significant role in vascular dysfunction and integrity, contributing to the attenuation of angiogenesis.
Conflict of interest statement
Figures






References
-
- Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. - PubMed
-
- Rolny C, Nilsson I, Magnusson P, et al. Platelet-derived growth factor receptor-beta promotes early endothelial cell differentiation. Blood. 2006;108:1877–1886. - PubMed
-
- von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312:623–629. - PubMed
-
- Fleming I. Vascular cytochrome p450 enzymes: physiology and pathophysiology. Trends Cardiovasc Med. 2008;18:20–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

