Multi-drug inhibition of the HER pathway in metastatic colorectal cancer: results of a phase I study of pertuzumab plus cetuximab in cetuximab-refractory patients
- PMID: 23568716
- PMCID: PMC3775976
- DOI: 10.1007/s10637-013-9956-5
Multi-drug inhibition of the HER pathway in metastatic colorectal cancer: results of a phase I study of pertuzumab plus cetuximab in cetuximab-refractory patients
Abstract
Purpose: Resistance to cetuximab, a monoclonal antibody against the epithelial growth factor receptor (EGFR), in colorectal cancer (CRC) may result from compensatory signaling through ErbB receptors, ErbB2/neu/HER2 (HER2) and ErbB3/HER3 (HER3). Pertuzumab is a monoclonal antibody that blocks HER2 hetero-dimerization; thus the combination of pertuzumab and cetuximab could possibly overcome cetuximab resistance.
Patients and methods: This single-arm, open-label, multicenter phase I/II study was designed to assess the safety and efficacy of pertuzumab and cetuximab in patients with cetuximab-resistant KRAS wild type metastatic CRC. Thirteen patients were enrolled and received cetuximab in combination with pertuzumab at several dose levels in a 3 + 3 design. Patients were assessed for dose-limiting toxicity (DLT) during the first cycle. A phase II portion was planned, but not initiated due to toxicity.
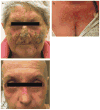
Results: Six of the thirteen patients (46 %) experienced DLTs, therefore the study was terminated early. Grade 3 or higher DLTs included dermatitis with desquamation and/or acneiform rash (n = 6), mucositis or stomatitis (n = 5), and diarrhea (n = 2). There was one Grade 5 event (myocardial infarction) attributed to underlying disease. Among the 13 patients, seven (54 %) were evaluable for response. The objective response rate was 14 %: one patient had a partial response lasting 6 months. Two patients had stable disease (29 %), and four had progressive disease (57 %). Median progression free survival was 2.1 months (95 % CI, 1.5-4.9) and median overall survival was 3.7 months (95 % CI, 1.6-7.9).
Conclusion: Combination pertuzumab and cetuximab in refractory CRC was associated with potential antitumor activity; however, the combination was not tolerable due to overlapping toxicities.
Conflict of interest statement
Dr. Hochster has received support from Genentech and Bristol-Myers Squibb. Dr. Wolpin has received support from Agensys/Astellas, Momenta Pharmaceuticals, Merrimack Pharmaceuticals, and Genentech. Dr. Lenz has served on Advisory Boards and received honoraria for lectures from Genentech. Dr. Bekaii-Saab has served as a consultant for Bristol Myers-Squibb and Genentech. Dr. Fuchs has received support from Genentech, Metamark Genetics, Sanofi, Amgen, Momenta Pharmaceuticals, Celgene, and Bayer.
Figures

References
-
- Saltz LB, Meropol NJ, Loehrer PJ, Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(7):1201–1208. doi: 10.1200/JCO.2004.10.182. - DOI - PubMed
-
- Martin-Martorell P, Rosello S, Rodriguez-Braun E, Chirivella I, Bosch A, Cervantes A. Biweekly cetuximab and irinotecan in advanced colorectal cancer patients progressing after at least one previous line of chemotherapy: results of a phase II single institution trial. British journal of cancer. 2008;99(3):455–458. doi: 10.1038/sj.bjc.6604530. - DOI - PMC - PubMed
-
- Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, Loos AH, Zubel A, Koralewski P. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(5):663–671. doi: 10.1200/JCO.2008.20.8397. - DOI - PubMed
-
- Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pinter T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. The New England journal of medicine. 2009;360(14):1408–1417. doi: 10.1056/NEJMoa0805019. - DOI - PubMed
-
- Van Cutsem E, Tejpar S, Vanbeckevoort D, Peeters M, Humblet Y, Gelderblom H, Vermorken JB, Viret F, Glimelius B, Gallerani E, Hendlisz A, Cats A, Moehler M, Sagaert X, Vlassak S, Schlichting M, Ciardiello F. Intrapatient Cetuximab Dose Escalation in Metastatic Colorectal Cancer According to the Grade of Early Skin Reactions: The Randomized EVEREST Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(23):2861–2868. doi: 10.1200/JCO.2011.40.9243. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

