Nuclear accumulation of cytosolic glyceraldehyde-3-phosphate dehydrogenase in cadmium-stressed Arabidopsis roots
- PMID: 23569110
- PMCID: PMC3641213
- DOI: 10.1104/pp.113.215194
Nuclear accumulation of cytosolic glyceraldehyde-3-phosphate dehydrogenase in cadmium-stressed Arabidopsis roots
Abstract
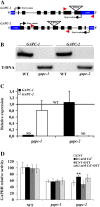
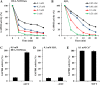
NAD-dependent glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is a ubiquitous enzyme involved in the glycolytic pathway. It has been widely demonstrated that mammalian GAPDH, in addition to its role in glycolysis, fulfills alternative functions mainly linked to its susceptibility to oxidative posttranslational modifications. Here, we investigated the responses of Arabidopsis (Arabidopsis thaliana) cytosolic GAPDH isoenzymes GAPC1 and GAPC2 to cadmium-induced stress in seedlings roots. GAPC1 was more responsive to cadmium than GAPC2 at the transcriptional level. In vivo, cadmium treatments induced different concomitant effects, including (1) nitric oxide accumulation, (2) cytosolic oxidation (e.g. oxidation of the redox-sensitive Green fluorescent protein2 probe), (3) activation of the GAPC1 promoter, (4) GAPC1 protein accumulation in enzymatically inactive form, and (5) strong relocalization of GAPC1 to the nucleus. All these effects were detected in the same zone of the root tip. In vitro, GAPC1 was inactivated by either nitric oxide donors or hydrogen peroxide, but no inhibition was directly provided by cadmium. Interestingly, nuclear relocalization of GAPC1 under cadmium-induced oxidative stress was stimulated, rather than inhibited, by mutating into serine the catalytic cysteine of GAPC1 (C155S), excluding an essential role of GAPC1 nitrosylation in the mechanism of nuclear relocalization, as found in mammalian cells. Although the function of GAPC1 in the nucleus is unknown, our results suggest that glycolytic GAPC1, through its high sensitivity to the cellular redox state, may play a role in oxidative stress signaling or protection in plants.
Figures





References
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Anderson LE, Ringenberg MR, Carol AA. (2004) Cytosolic glyceraldehyde-3-P dehydrogenase and the B subunit of the chloroplast enzyme are present in the pea leaf nucleus. Protoplasma 223: 33–43 - PubMed
-
- Apel K, Hirt H. (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399 - PubMed
-
- Astier J, Rasul S, Koen E, Manzoor H, Besson-Bard A, Lamotte O, Jeandroz S, Durner J, Lindermayr C, Wendehenne D. (2011) S-nitrosylation: an emerging post-translational protein modification in plants. Plant Sci 181: 527–533 - PubMed
-
- Bedhomme M, Adamo M, Marchand CH, Couturier J, Rouhier N, Lemaire SD, Zaffagnini M, Trost P. (2012) Glutathionylation of cytosolic glyceraldehyde-3-phosphate dehydrogenase from the model plant Arabidopsis thaliana is reversed by both glutaredoxins and thioredoxins in vitro. Biochem J 445: 337–347 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

