Molecular characterization of the carboxypeptidase B1 of Anopheles stephensi and its evaluation as a target for transmission-blocking vaccines
- PMID: 23569111
- PMCID: PMC3676044
- DOI: 10.1128/IAI.01331-12
Molecular characterization of the carboxypeptidase B1 of Anopheles stephensi and its evaluation as a target for transmission-blocking vaccines
Abstract
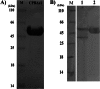
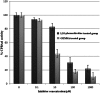
Malaria is one of the most important infectious diseases in the world, and it has many economic and social impacts on populations, especially in poor countries. Transmission-blocking vaccines (TBVs) are valuable tools for malaria eradication. A study on Anopheles gambiae revealed that polyclonal antibodies to carboxypeptidase B1 of A. gambiae can block sexual parasite development in the mosquito midgut. Hence, it was introduced as a TBV target in regions where A. gambiae is the main malaria vector. However, in Iran and neighboring countries as far as China, the main malaria vector is Anopheles stephensi. Also, the genome of this organism has not been sequenced yet. Therefore, in this study, carboxypeptidase B1 of A. stephensi was characterized by genomic and proteomic approaches. Furthermore, its expression pattern after ingestion of Plasmodium falciparum gametocytes and the effect of anti-CPBAs1 antibodies on sexual parasite development were evaluated. Our results revealed that the cpbAs1 expression level was increased after ingestion of the mature gametocytes of P. falciparum and that anti-CPBAs1 directed antibodies could significantly reduce the mosquito infection rate in the test group compared with the control group. Therefore, according to our findings and with respect to the high similarity of carboxypeptidase enzymes between the two main malaria vectors in Africa (A. gambiae) and Asia (A. stephensi) and the presence of other sympatric vectors, CPBAs1 could be introduced as a TBV candidate in regions where A. stephensi is the main malaria vector, and this will broaden the scope for the potential wider application of CPBAs1 antigen homologs/orthologs.
Figures





References
-
- malERA Consultative Group on Vaccines 2011. A research agenda for malaria eradication: vaccines. PLoS Med. 8:e1000398 doi:10.1371/journal.pmed.1000398 - DOI - PMC - PubMed
-
- Hisaeda H, Yasutomo K. 2002. Development of malaria vaccines that block transmission of parasites by mosquito vectors. J. Med. Invest. 49:118–123 - PubMed
-
- Kaslow DC. 1997. Transmission-blocking vaccines: uses and current status of development. Int. J. Parasitol. 27:183–189 - PubMed
-
- Kaslow DC. 2002. Transmission-blocking vaccines. Chem. Immunol. 80:287–307 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

