Conversion of mature human β-cells into glucagon-producing α-cells
- PMID: 23569174
- PMCID: PMC3712074
- DOI: 10.2337/db12-1001
Conversion of mature human β-cells into glucagon-producing α-cells
Abstract
Conversion of one terminally differentiated cell type into another (or transdifferentiation) usually requires the forced expression of key transcription factors. We examined the plasticity of human insulin-producing β-cells in a model of islet cell aggregate formation. Here, we show that primary human β-cells can undergo a conversion into glucagon-producing α-cells without introduction of any genetic modification. The process occurs within days as revealed by lentivirus-mediated β-cell lineage tracing. Converted cells are indistinguishable from native α-cells based on ultrastructural morphology and maintain their α-cell phenotype after transplantation in vivo. Transition of β-cells into α-cells occurs after β-cell degranulation and is characterized by the presence of β-cell-specific transcription factors Pdx1 and Nkx6.1 in glucagon(+) cells. Finally, we show that lentivirus-mediated knockdown of Arx, a determinant of the α-cell lineage, inhibits the conversion. Our findings reveal an unknown plasticity of human adult endocrine cells that can be modulated. This endocrine cell plasticity could have implications for islet development, (patho)physiology, and regeneration.
Figures
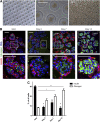
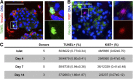



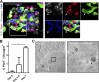

References
-
- Brissova M, Fowler MJ, Nicholson WE, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 2005;53:1087–1097 - PubMed
-
- Courtney M, Pfeifer A, Al-Hasani K, et al. In vivo conversion of adult α-cells into β-like cells: a new research avenue in the context of type 1 diabetes. Diabetes Obes Metab 2011;13(Suppl. 1):47–52 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

