Concentrations of insulin glargine and its metabolites during long-term insulin therapy in type 2 diabetic patients and comparison of effects of insulin glargine, its metabolites, IGF-I, and human insulin on insulin and igf-I receptor signaling
- PMID: 23569175
- PMCID: PMC3712030
- DOI: 10.2337/db12-1773
Concentrations of insulin glargine and its metabolites during long-term insulin therapy in type 2 diabetic patients and comparison of effects of insulin glargine, its metabolites, IGF-I, and human insulin on insulin and igf-I receptor signaling
Abstract
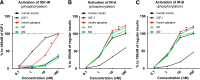
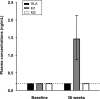
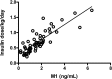
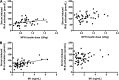
We investigated 1) the ability of purified glargine (GLA), metabolites 1 (M1) and 2 (M2), IGF-I, and NPH insulin to activate the insulin receptor (IR)-A and IR-B and IGF-I receptor (IGF-IR) in vitro; 2) plasma concentrations of GLA, M1, and M2 during long-term insulin therapy in type 2 diabetic patients; and 3) IR-A and IR-B activation in vitro induced by serum from patients treated with GLA or NPH insulin. A total of 104 patients (age 56.3 ± 0.8 years, BMI 31.4 ± 0.5 kg/m(2), and A1C 9.1 ± 0.1% [mean ± SE]) were randomized to GLA or NPH insulin therapy for 36 weeks. Plasma concentrations of GLA, M1, and M2 were determined by liquid chromatography-tandem mass spectrometry assay. IR-A, IR-B, and IGF-IR autophosphorylation was induced by purified hormones or serum by kinase receptor activation assays. In vitro, M1 induced comparable IR-A, IR-B, and IGF-IR autophosphorylation (activation) as NPH insulin. After 36 weeks, M1 increased from undetectable (<0.2 ng/mL) to 1.5 ng/mL (0.9-2.1), while GLA and M2 remained undetectable. GLA dose correlated with M1 (r = 0.84; P < 0.001). Serum from patients treated with GLA or NPH insulin induced similar IR-A and IR-B activation. These data suggest that M1 rather than GLA mediates GLA effects and that compared with NPH insulin, GLA does not increase IGF-IR signaling during long-term insulin therapy in type 2 diabetes.
Figures
References
-
- Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009;52:1766–1777 - PubMed
-
- Jonasson JM, Ljung R, Talbäck M, Haglund B, Gudbjörnsdòttir S, Steineck G. Insulin glargine use and short-term incidence of malignancies-a population-based follow-up study in Sweden. Diabetologia 2009;52:1745–1754 - PubMed
-
- Kurtzhals P, Schäffer L, Sørensen A, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 2000;49:999–1005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

