The variable domain of a plant calcium-dependent protein kinase (CDPK) confers subcellular localization and substrate recognition for NADPH oxidase
- PMID: 23569203
- PMCID: PMC3656289
- DOI: 10.1074/jbc.M112.448910
The variable domain of a plant calcium-dependent protein kinase (CDPK) confers subcellular localization and substrate recognition for NADPH oxidase
Abstract
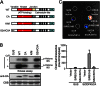
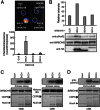
Calcium-dependent protein kinases (CDPKs) are Ca(2+) sensors that regulate diverse biological processes in plants and apicomplexans. However, how CDPKs discriminate specific substrates in vivo is still largely unknown. Previously, we found that a potato StCDPK5 is dominantly localized to the plasma membrane and activates the plasma membrane NADPH oxidase (RBOH; for respiratory burst oxidase homolog) StRBOHB by direct phosphorylation of the N-terminal region. Here, we report the contribution of the StCDPK5 N-terminal variable (V) domain to activation of StRBOHB in vivo using heterologous expression system in Nicotiana benthamiana. Mutations of N-terminal myristoylation and palmitoylation sites in the V domain eliminated the predominantly plasma membrane localization and the capacity of StCDPK5 to activate StRBOHB in vivo. A tomato SlCDPK2, which also contains myristoylation and palmitoylation sites in its N terminus, phosphorylated StRBOHB in vitro but not in vivo. Functional domains responsible for activation and phosphorylation of StRBOHB were identified by swapping regions for each domain between StCDPK5 and SlCDPK2. The substitution of the V domain of StCDPK5 with that of SlCDPK2 abolished the activation and phosphorylation abilities of StRBOHB in vivo and relocalized the chimeric CDPK to the trans-Golgi network, as observed for SlCDPK2. Conversely, SlCDPK2 substituted with the V domain of StCDPK5 localized to the plasma membrane and activated StRBOHB. These results suggest that the V domains confer substrate specificity in vivo by dictating proper subcellular localization of CDPKs.
Keywords: Calcium-dependent Protein Kinase; NADPH Oxidase; Phosphorylation; Plant Defense; Plant Immunity; Reactive Oxygen Species (ROS); Signal Transduction.
Figures



References
-
- Seet B. T., Dikic I., Zhou M. M., Pawson T. (2006) Reading protein modifications with interaction domains. Nat. Rev. Mol. Cell Biol. 7, 473–483 - PubMed
-
- Eckhart W., Hutchinson M. A., Hunter T. (1979) An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell 18, 925–933 - PubMed
-
- Sharrocks A. D., Yang S. H., Galanis A. (2000) Docking domains and substrate-specificity determination for MAP kinases. Trends Biochem. Sci. 25, 448–453 - PubMed
-
- Ishihama N., Yoshioka H. (2012) Post-translational regulation of WRKY transcription factors in plant immunity. Curr. Opin. Plant Biol. 15, 431–437 - PubMed
-
- Ubersax J. A., Woodbury E. L., Quang P. N., Paraz M., Blethrow J. D., Shah K., Shokat K. M., Morgan D. O. (2003) Targets of the cyclin-dependent kinase Cdk1. Nature 425, 859–864 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

