Regulation of Torsin ATPases by LAP1 and LULL1
- PMID: 23569223
- PMCID: PMC3637692
- DOI: 10.1073/pnas.1300676110
Regulation of Torsin ATPases by LAP1 and LULL1
Abstract
TorsinA is a membrane-associated AAA+ (ATPases associated with a variety of cellular activities) ATPase implicated in primary dystonia, an autosomal-dominant movement disorder. We reconstituted TorsinA and its cofactors in vitro and show that TorsinA does not display ATPase activity in isolation; ATP hydrolysis is induced upon association with LAP1 and LULL1, type II transmembrane proteins residing in the nuclear envelope and endoplasmic reticulum. This interaction requires TorsinA to be in the ATP-bound state, and can be attributed to the luminal domains of LAP1 and LULL1. This ATPase activator function controls the activities of other members of the Torsin family in distinct fashion, leading to an acceleration of the hydrolysis step by up to two orders of magnitude. The dystonia-causing mutant of TorsinA is defective in this activation mechanism, suggesting a loss-of-function mechanism for this congenital disorder.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

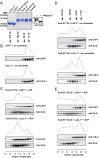
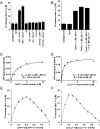
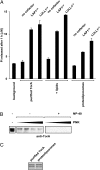

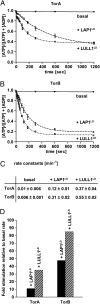
References
-
- Ozelius LJ, et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17(1):40–48. - PubMed
-
- Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9(1):27–43. - PubMed
-
- Hanson PI, Whiteheart SW. AAA+ proteins: Have engine, will work. Nat Rev Mol Cell Biol. 2005;6(7):519–529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

