Bacillus subtilis biofilm induction by plant polysaccharides
- PMID: 23569226
- PMCID: PMC3637697
- DOI: 10.1073/pnas.1218984110
Bacillus subtilis biofilm induction by plant polysaccharides
Abstract
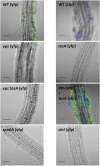
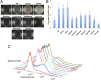
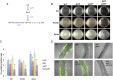
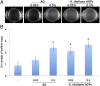
Bacillus subtilis is a plant-beneficial Gram-positive bacterium widely used as a biofertilizer. However, relatively little is known regarding the molecular processes underlying this bacterium's ability to colonize roots. In contrast, much is known about how this bacterium forms matrix-enclosed multicellular communities (biofilms) in vitro. Here, we show that, when B. subtilis colonizes Arabidopsis thaliana roots it forms biofilms that depend on the same matrix genes required in vitro. B. subtilis biofilm formation was triggered by certain plant polysaccharides. These polysaccharides served as a signal for biofilm formation transduced via the kinases controlling the phosphorylation state of the master regulator Spo0A. In addition, plant polysaccharides are used as a source of sugars for the synthesis of the matrix exopolysaccharide. The bacterium's response to plant polysaccharides was observed across several different strains of the species, some of which are known to have beneficial effects on plants. These observations provide evidence that biofilm genes are crucial for Arabidopsis root colonization by B. subtilis and provide insights into how matrix synthesis may be triggered by this plant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Hiltner L. Über neuere Erfahrungen und Probleme auf dem Gebiete der Bodenbakteriologie unter bessonderer Berücksichtigung der Gründung und Brache [About our experiences and problems in the area of soil bacteriology under special regards of cultivated and uncultivated land] Arbeiten der Deutschen Landwirtschaftlichen Gesellschaft. 1904;98:59–78.
-
- Kloepper J, Leong J, Teintze M, Schroth M. Enhanced plant growth by siderophore produced by plant growth-promoting rhizobacteria. Nature. 1980;286(5776):885–886.
-
- Lugtenberg B, Kamilova F. Plant-growth-promoting rhizobacteria. Annu Rev Microbiol. 2009;63:541–556. - PubMed
-
- Vessey J. Plant growth promoting rhizobacteria as biofertiliers. Plant Soil. 2003;255(2):571–586.
-
- Berg G. Plant-microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl Microbiol Biotechnol. 2009;84(1):11–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

