Newly characterized Golgi-localized family of proteins is involved in calcium and pH homeostasis in yeast and human cells
- PMID: 23569283
- PMCID: PMC3637739
- DOI: 10.1073/pnas.1219871110
Newly characterized Golgi-localized family of proteins is involved in calcium and pH homeostasis in yeast and human cells
Abstract
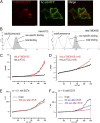
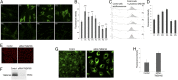
Defects in the human protein TMEM165 are known to cause a subtype of Congenital Disorders of Glycosylation. Transmembrane protein 165 (TMEM165) belongs to an uncharacterized family of membrane proteins called Uncharacterized Protein Family 0016, which are well conserved throughout evolution and share characteristics reminiscent of the cation/Ca(2+) exchanger superfamily. Gcr1 dependent translation factor 1 (Gdt1p), the budding yeast member of this family, contributes to Ca(2+) homeostasis via an uncharacterized Ca(2+) transport pathway localized in the Golgi apparatus. The gdt1Δ mutant was found to be sensitive to high concentrations of Ca(2+), and interestingly, this sensitivity was suppressed by expression of TMEM165, the human ortholog of Gdt1p, indicating conservation of function among the members of this family. Patch-clamp analyses on human cells indicated that TMEM165 expression is linked to Ca(2+) ion transport. Furthermore, defects in TMEM165 affected both Ca(2+) and pH homeostasis. Based on these results, we propose that Gdt1p and TMEM165 could be members of a unique family of Golgi-localized Ca(2+)/H(+) antiporters and that modification of the Golgi Ca(2+) and pH balance could explain the glycosylation defects observed in TMEM165-deficient patients.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Wu X, et al. Mutation of the COG complex subunit gene COG7 causes a lethal congenital disorder. Nat Med. 2004;10(5):518–523. - PubMed
-
- Foulquier F, et al. A new inborn error of glycosylation due to a Cog8 deficiency reveals a critical role for the Cog1-Cog8 interaction in COG complex formation. Hum Mol Genet. 2007;16(7):717–730. - PubMed
-
- Reynders E, Foulquier F, Annaert W, Matthijs G. How Golgi glycosylation meets and needs trafficking: The case of the COG complex. Glycobiology. 2011;21(7):853–863. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

