Modified toxicity probability interval design: a safer and more reliable method than the 3 + 3 design for practical phase I trials
- PMID: 23569307
- PMCID: PMC3641699
- DOI: 10.1200/JCO.2012.45.7903
Modified toxicity probability interval design: a safer and more reliable method than the 3 + 3 design for practical phase I trials
Abstract
The 3 + 3 design is the most common choice among clinicians for phase I dose-escalation oncology trials. In recent reviews, more than 95% of phase I trials have been based on the 3 + 3 design. Given that it is intuitive and its implementation does not require a computer program, clinicians can conduct 3 + 3 dose escalations in practice with virtually no logistic cost, and trial protocols based on the 3 + 3 design pass institutional review board and biostatistics reviews quickly. However, the performance of the 3 + 3 design has rarely been compared with model-based designs in simulation studies with matched sample sizes. In the vast majority of statistical literature, the 3 + 3 design has been shown to be inferior in identifying true maximum-tolerated doses (MTDs), although the sample size required by the 3 + 3 design is often orders-of-magnitude smaller than model-based designs. In this article, through comparative simulation studies with matched sample sizes, we demonstrate that the 3 + 3 design has higher risks of exposing patients to toxic doses above the MTD than the modified toxicity probability interval (mTPI) design, a newly developed adaptive method. In addition, compared with the mTPI design, the 3 + 3 design does not yield higher probabilities in identifying the correct MTD, even when the sample size is matched. Given that the mTPI design is equally transparent, costless to implement with free software, and more flexible in practical situations, we highly encourage its adoption in early dose-escalation studies whenever the 3 + 3 design is also considered. We provide free software to allow direct comparisons of the 3 + 3 design with other model-based designs in simulation studies with matched sample sizes.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

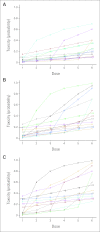
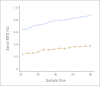
References
-
- Storer BE. An evaluation of phase I clinical trials designs in the continuous dose-response setting. Stat Med. 2001;20:2399–2408. - PubMed
-
- Storer BE. Design and analysis of phase I clinical trials. Biometrics. 1989;45:925–937. - PubMed
-
- Rogatko A, Schoeneck D, Jonas W, et al. Translation of innovative designs into phase I trials. J Clin Oncol. 2007;25:4982–4986. - PubMed
-
- Ji Y, Li Y, Nebiyou Bekele B. Dose-finding in phase I clinical trials based on toxicity probability intervals. Clin Trials. 2007;4:235–244. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

