Antigen delivery to early endosomes eliminates the superiority of human blood BDCA3+ dendritic cells at cross presentation
- PMID: 23569326
- PMCID: PMC3646496
- DOI: 10.1084/jem.20121251
Antigen delivery to early endosomes eliminates the superiority of human blood BDCA3+ dendritic cells at cross presentation
Abstract
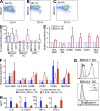
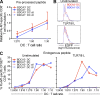
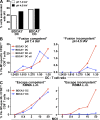
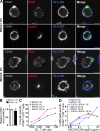
Human BDCA3(+) dendritic cells (DCs), the proposed equivalent to mouse CD8α(+) DCs, are widely thought to cross present antigens on MHC class I (MHCI) molecules more efficiently than other DC populations. If true, it is unclear whether this reflects specialization for cross presentation or a generally enhanced ability to present antigens on MHCI. We compared presentation by BDCA3(+) DCs with BDCA1(+) DCs using a quantitative approach whereby antigens were targeted to distinct intracellular compartments by receptor-mediated internalization. As expected, BDCA3(+) DCs were superior at cross presentation of antigens delivered to late endosomes and lysosomes by uptake of anti-DEC205 antibody conjugated to antigen. This difference may reflect a greater efficiency of antigen escape from BDCA3(+) DC lysosomes. In contrast, if antigens were delivered to early endosomes through CD40 or CD11c, BDCA1(+) DCs were as efficient at cross presentation as BDCA3(+) DCs. Because BDCA3(+) DCs and BDCA1(+) DCs were also equivalent at presenting peptides and endogenously synthesized antigens, BDCA3(+) DCs are not likely to possess mechanisms for cross presentation that are specific to this subset. Thus, multiple DC populations may be comparably effective at presenting exogenous antigens to CD8(+) T cells as long as the antigen is delivered to early endocytic compartments.
Figures




References
-
- Bachem A., Güttler S., Hartung E., Ebstein F., Schaefer M., Tannert A., Salama A., Movassaghi K., Opitz C., Mages H.W., et al. 2010. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J. Exp. Med. 207:1273–1281 10.1084/jem.20100348 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

