Phase I/II trial of adeno-associated virus-mediated alpha-glucosidase gene therapy to the diaphragm for chronic respiratory failure in Pompe disease: initial safety and ventilatory outcomes
- PMID: 23570273
- PMCID: PMC3689178
- DOI: 10.1089/hum.2012.250
Phase I/II trial of adeno-associated virus-mediated alpha-glucosidase gene therapy to the diaphragm for chronic respiratory failure in Pompe disease: initial safety and ventilatory outcomes
Abstract
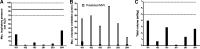
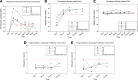
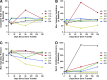
Pompe disease is an inherited neuromuscular disease caused by deficiency of lysosomal acid alpha-glucosidase (GAA) leading to glycogen accumulation in muscle and motoneurons. Cardiopulmonary failure in infancy leads to early mortality, and GAA enzyme replacement therapy (ERT) results in improved survival, reduction of cardiac hypertrophy, and developmental gains. However, many children have progressive ventilatory insufficiency and need additional support. Preclinical work shows that gene transfer restores phrenic neural activity and corrects ventilatory deficits. Here we present 180-day safety and ventilatory outcomes for five ventilator-dependent children in a phase I/II clinical trial of AAV-mediated GAA gene therapy (rAAV1-hGAA) following intradiaphragmatic delivery. We assessed whether rAAV1-hGAA results in acceptable safety outcomes and detectable functional changes, using general safety measures, immunological studies, and pulmonary functional testing. All subjects required chronic, full-time mechanical ventilation because of respiratory failure that was unresponsive to both ERT and preoperative muscle-conditioning exercises. After receiving a dose of either 1×10(12) vg (n=3) or 5×10(12) vg (n=2) of rAAV1-hGAA, the subjects' unassisted tidal volume was significantly larger (median [interquartile range] 28.8% increase [15.2-35.2], p<0.05). Further, most patients tolerated appreciably longer periods of unassisted breathing (425% increase [103-851], p=0.08). Gene transfer did not improve maximal inspiratory pressure. Expected levels of circulating antibodies and no T-cell-mediated immune responses to the vector (capsids) were observed. One subject demonstrated a slight increase in anti-GAA antibody that was not considered clinically significant. These results indicate that rAAV1-hGAA was safe and may lead to modest improvements in volitional ventilatory performance measures. Evaluation of the next five patients will determine whether earlier intervention can further enhance the functional benefit.
Figures


References
-
- Allen J. Pulmonary complications of neuromuscular disease: a respiratory mechanics perspective. Paediatr. Respir. Rev. 2010;11:18–23. - PubMed
-
- American Thoracic Society/European Respiratory Society. ATS/ERS Statement on respiratory muscle testing. Am. J. Respir. Crit. Care Med. 2002;166:518–624. - PubMed
-
- Baydur A. Kanel G. Tracheobronchomalacia and tracheal hemorrhage in patients with Duchenne muscular dystrophy receiving long-term ventilation with uncuffed tracheostomies. Chest. 2003;123:1307–1311. - PubMed
-
- Bolton C.F. Grand'Maison F. Parkes A. Shkrum M. Needle electromyography of the diaphragm. Muscle Nerve. 1992;15:678–681. - PubMed
-
- Brantly M.L. Spencer L.T. Humphries M., et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 alphal-antitrypsin (AAT) vector in AAT-deficient adults. Hum. Gene Ther. 2006;17:1177–1186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

