The effects of inpatient hybrid closed-loop therapy initiated within 1 week of type 1 diabetes diagnosis
- PMID: 23570538
- PMCID: PMC3643224
- DOI: 10.1089/dia.2013.0002
The effects of inpatient hybrid closed-loop therapy initiated within 1 week of type 1 diabetes diagnosis
Abstract
Background: This article describes our experience with inpatient hybrid closed-loop control (HCLC) initiated shortly after the diagnosis of type 1 diabetes in a randomized trial designed to assess the effectiveness of inpatient HCLC followed by outpatient sensor-augmented pump (SAP) therapy on the preservation of β-cell function.
Subjects and methods: Forty-eight individuals with newly diagnosed type 1 diabetes and positive pancreatic autoantibodies (7.8-37.7 years old) received inpatient HCLC therapy for up to 93 h, initiated within 7 days of diagnosis.
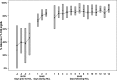
Results: On initiation of HCLC, mean glucose concentration was 240±100 mg/dL. During the first day of HCLC, median of the participant's mean glucose concentrations fell rapidly to 146 mg/dL, a level of control that was sustained on Days 2 and 3 (138 mg/dL and 139 mg/dL, respectively). By Day 3, the median percentage of glucose values >250 and <60 mg/dL was <1%. During the first 2 weeks of SAP treatment at home, the median participant mean glucose level was 126 mg/dL (interquartile range, 117, 137 mg/dL), and the median percentage of values between 71 and 180 mg/dL was 85% (interquartile range, 80%, 90%).
Conclusions: Inpatient HCLC followed by outpatient SAP therapy can provide a safe and effective means to rapidly reverse glucose toxicity and establish near-normal glycemic control in patients with newly diagnosed type 1 diabetes.
Figures

References
-
- Shah SC. Malone JI. Simpson NE. A randomized trial of intensive insulin therapy in newly diagnosed insulin-dependent diabetes mellitus. N Engl J Med. 1989;320:550–554. - PubMed
-
- Kordonouri O. Pankowska E. Rami B. Kapellen T. Coutant R. Hartmann R. Lange K. Knip M. Danne T. Sensor-augmented pump therapy from the diagnosis of childhood type 1 diabetes: results of the Paediatric Onset Study (ONSET) after 12 months of treatment. Diabetologia. 2010;53:2487–2495. - PubMed
-
- Steffes MW. Sibley S. Jackson M. Thomas W. β-Cell function and the development of diabetes-related complications in the Diabetes Control and Complications Trial. Diabetes Care. 2003;26:832–836. - PubMed
-
- The Diabetes Control and Complications Trial Research Group. Effect of intensive therapy on residual β-cell function in patients with type 1 diabetes in the Diabetes Control and Complications Trial. A randomized, controlled trial. Ann Intern Med. 1998;128:517–523. - PubMed
-
- Bergenstal RM. Tamborlane WV. Ahmann A. Buse JB. Dailey G. Davis SN. Joyce C. Peoples T. Perkins BA. Welsh JB. Willi SM. Wood MA the STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363:311–320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1TR000445/TR/NCATS NIH HHS/United States
- HD41890-10/HD/NICHD NIH HHS/United States
- U01DK06104211/DK/NIDDK NIH HHS/United States
- UL1 RR025744/RR/NCRR NIH HHS/United States
- 5U01DK085465-04/DK/NIDDK NIH HHS/United States
- U01DK085509/DK/NIDDK NIH HHS/United States
- 5U01DK085466-05/DK/NIDDK NIH HHS/United States
- HD41908-10/HD/NICHD NIH HHS/United States
- U01DK085505-02/DK/NIDDK NIH HHS/United States
- UL1 RR024139/RR/NCRR NIH HHS/United States
- 1UL1 RR025780/RR/NCRR NIH HHS/United States
- SUL1 RR025744/RR/NCRR NIH HHS/United States
- UL1 TR001085/TR/NCATS NIH HHS/United States
- HD41906-10/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

