Short-term recognition memory correlates with regional CNS expression of microRNA-138 in mice
- PMID: 23570889
- PMCID: PMC3660985
- DOI: 10.1016/j.jagp.2012.09.005
Short-term recognition memory correlates with regional CNS expression of microRNA-138 in mice
Abstract
Objectives: We hypothesized that microRNA (miR) expression may be involved in memory function because it controls local protein translation at synapses and dendritic spines.
Design: Case-control animal study.
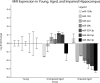
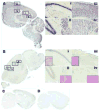
Methods: We assessed the miR repertoire in the hippocampus of young, 6-month-old (N = 18) mice compared with aged, 26-month-old (N = 23) mice and compared miR quantity to memory scores as determined by the novel object recognition task. We performed a histological brain regional analysis of miR-138, acyl protein thioesterase 1 (APT1) mRNA, and APT1 protein.
Results: We found that higher miR-138 expression in the mouse hippocampus is correlated with better memory performance. We also found that APT1 (a depalmytoylation enzyme expressed at dendritic spines whose translation is controlled by miR-138) mRNA is increased in the mouse hippocampal CA1 and dentate gyrus in aged mice compared with young mice, but not in mice with memory impairment. We found APT1 protein distribution to be lower in cells with high miR-138 expression.
Conclusions: These results suggest that increased miR-138 is associated with better memory and increased APT1 gene transcription occurs with aging. The role of miR-138 and APT1 protein function in memory and aging warrants further investigation.
Copyright © 2013 American Association for Geriatric Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures

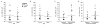





References
-
- Ashraf SI, Kunes S. A trace of silence: memory and microRNA at the synapse. Curr Opin Neurobiol. 2006;16:535–539. - PubMed
-
- Bramham CR, Wells DG. Dendritic mRNA: transport, translation and function. Nat Rev Neurosci. 2007;8:776–789. - PubMed
-
- Martin KC, Barad M, Kandel ER. Local protein synthesis and its role in synapse-specific plasticity. Curr Opin Neurobiol. 2000;10:587–592. - PubMed
-
- Ostroff LE, Fiala JC, Allwardt B, et al. Polyribosomes redistribute from dendritic shafts into spines with enlarged synapses during LTP in developing rat hippocampal slices. Neuron. 2002;35:535–545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH019934/MH/NIMH NIH HHS/United States
- T32 MH019934/MH/NIMH NIH HHS/United States
- DA026306/DA/NIDA NIH HHS/United States
- AI36214/AI/NIAID NIH HHS/United States
- MH080002/MH/NIMH NIH HHS/United States
- P30 AI036214/AI/NIAID NIH HHS/United States
- MH074697/MH/NIMH NIH HHS/United States
- MH062512/MH/NIMH NIH HHS/United States
- P50 DA026306/DA/NIDA NIH HHS/United States
- R03 DA031591/DA/NIDA NIH HHS/United States
- R01 MH074697/MH/NIMH NIH HHS/United States
- P30 MH080002/MH/NIMH NIH HHS/United States
- DA031591/DA/NIDA NIH HHS/United States
- P30 MH062512/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

