Early inhibition of MMP activity in ischemic rat brain promotes expression of tight junction proteins and angiogenesis during recovery
- PMID: 23571276
- PMCID: PMC3705440
- DOI: 10.1038/jcbfm.2013.56
Early inhibition of MMP activity in ischemic rat brain promotes expression of tight junction proteins and angiogenesis during recovery
Abstract
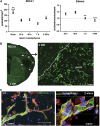
In cerebral ischemia, matrix metalloproteinases (MMPs) have a dual role by acutely disrupting tight junction proteins (TJPs) in the blood-brain barrier (BBB) and chronically promoting angiogenesis. Since TJP remodeling of the neurovascular unit (NVU) is important in recovery and early inhibition of MMPs is neuroprotective, we hypothesized that short-term MMP inhibition would reduce infarct size and promote angiogenesis after ischemia. Adult spontaneously hypertensive rats had a transient middle cerebral artery occlusion with reperfusion. At the onset of ischemia, they received a single dose of the MMP inhibitor, GM6001. They were studied at multiple times up to 4 weeks with immunohistochemistry, biochemistry, and magnetic resonance imaging (MRI). We observed newly formed vessels in peri-infarct regions at 3 weeks after reperfusion. Dynamic contrast-enhanced MRI showed BBB opening in new vessels. Along with the new vessels, pericytes expressed zonula occludens-1 (ZO-1) and MMP-3, astrocytes expressed ZO-1, occludin, and MMP-2, while endothelial cells expressed claudin-5. The GM6001, which reduced tissue loss at 3 to 4 weeks, significantly increased new vessel formation with expression of TJPs and MMPs. Our results show that pericytes and astrocytes act spatiotemporally, contributing to extraendothelial TJP formation, and that MMPs are involved in BBB restoration during recovery. Early MMP inhibition benefits neurovascular remodeling after stroke.
Figures






References
-
- Hermann DM, Zechariah A. Implications of vascular endothelial growth factor for postischemic neurovascular remodeling. J Cereb Blood Flow Metab. 2009;29:1620–1643. - PubMed
-
- Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Some remarks on the growth-rate and angiogenesis of microvessels in ischemic stroke. Morphometric and immunocytochemical studies. Patol Pol. 1993;44:203–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

