Chronic dry eye disease is principally mediated by effector memory Th17 cells
- PMID: 23571503
- PMCID: PMC3732510
- DOI: 10.1038/mi.2013.20
Chronic dry eye disease is principally mediated by effector memory Th17 cells
Abstract
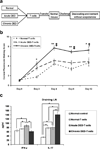
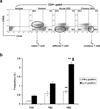
Recent experimental and clinical data suggest that there is a link between dry eye disease (DED) and T-cell-mediated immunity. However, whether these immune responses are a consequence or cause of ocular surface inflammation remains to be determined. Thus far, only models of acute DED have been used to derive experimental data. This is in contrast to clinical DED which usually presents as a chronic disease. In the present study, using a murine model of chronic DED, it was established that the chronic phase of the disease is accompanied by T helper type 17 (Th17) responses at the ocular surface and that a significant memory T-cell population can be recovered from chronic DED. This memory response is predominantly mediated by Th17 cells. Moreover, adoptive transfer of this memory T-cell population was shown to induce more severe and rapidly progressing DED than did the adoptive transfer of its effector or naive counterparts. Not only do these results clearly demonstrate that effector memory Th17 cells are primarily responsible for maintaining the chronic and relapsing course of DED, but they also highlight a potentially novel therapeutic strategy for targeting memory immune responses in patients with DED.
Conflict of interest statement
Conflict of interest: None.
Figures


References
-
- Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am. J. Ophthalmol. 2003;136:318–326. - PubMed
-
- Subcommittee of the international Dry Eye Workshop. The definition and classification of dry eye disease: report of the Definition and Classification. Ocul. Surf. 2007;5:75–92. - PubMed
-
- Stern ME, et al. Conjunctival T cell subpopulations in Sjögren's and non-Sjögren's patients with dry eye. Invest. Ophthalmol. Vis. Sci. 2002;43:2609–2614. - PubMed
-
- Kunert KS, Tisdale AS, Stern ME, Smith JA, Gipson IK. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch. Ophthalmol. 2000;118:1489–1496. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

