Pharmacokinetics and pharmacodynamics of fluconazole for cryptococcal meningoencephalitis: implications for antifungal therapy and in vitro susceptibility breakpoints
- PMID: 23571544
- PMCID: PMC3716186
- DOI: 10.1128/AAC.00216-13
Pharmacokinetics and pharmacodynamics of fluconazole for cryptococcal meningoencephalitis: implications for antifungal therapy and in vitro susceptibility breakpoints
Abstract
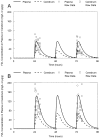
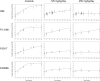
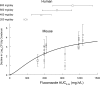
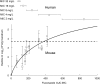
Fluconazole is frequently the only antifungal agent that is available for induction therapy for cryptococcal meningitis. There is relatively little understanding of the pharmacokinetics and pharmacodynamics (PK-PD) of fluconazole in this setting. PK-PD relationships were estimated with 4 clinical isolates of Cryptococcus neoformans. MICs were determined using Clinical and Laboratory Standards Institute (CLSI) methodology. A nonimmunosuppressed murine model of cryptococcal meningitis was used. Mice received two different doses of fluconazole (125 mg/kg of body weight/day and 250 mg/kg of body weight/day) orally for 9 days; a control group of mice was not given fluconazole. Fluconazole concentrations in plasma and in the cerebrum were determined using high-performance liquid chromatography (HPLC). The cryptococcal density in the brain was estimated using quantitative cultures. A mathematical model was fitted to the PK-PD data. The experimental results were extrapolated to humans (bridging study). The PK were linear. A dose-dependent decline in fungal burden was observed, with near-maximal activity evident with dosages of 250 mg/kg/day. The MIC was important for understanding the exposure-response relationships. The mean AUC/MIC ratio associated with stasis was 389. The results of the bridging study suggested that only 66.7% of patients receiving 1,200 mg/kg would achieve or exceed an AUC/MIC ratio of 389. The potential breakpoints for fluconazole against Cryptococcus neoformans follow: susceptible, ≤ 2 mg/liter; resistant, >2 mg/liter. Fluconazole may be an inferior agent for induction therapy because many patients cannot achieve the pharmacodynamic target. Clinical breakpoints are likely to be significantly lower than epidemiological cutoff values. The MIC may guide the appropriate use of fluconazole. If fluconazole is the only option for induction therapy, then the highest possible dose should be used.
Figures
Similar articles
-
Fluconazole Monotherapy Is a Suboptimal Option for Initial Treatment of Cryptococcal Meningitis Because of Emergence of Resistance.mBio. 2019 Dec 3;10(6):e02575-19. doi: 10.1128/mBio.02575-19. mBio. 2019. PMID: 31796539 Free PMC article.
-
Repurposing and Reformulation of the Antiparasitic Agent Flubendazole for Treatment of Cryptococcal Meningoencephalitis, a Neglected Fungal Disease.Antimicrob Agents Chemother. 2018 Mar 27;62(4):e01909-17. doi: 10.1128/AAC.01909-17. Print 2018 Apr. Antimicrob Agents Chemother. 2018. PMID: 29311092 Free PMC article.
-
Experimental Models of Short Courses of Liposomal Amphotericin B for Induction Therapy for Cryptococcal Meningitis.Antimicrob Agents Chemother. 2017 May 24;61(6):e00090-17. doi: 10.1128/AAC.00090-17. Print 2017 Jun. Antimicrob Agents Chemother. 2017. PMID: 28320715 Free PMC article.
-
Interpretive breakpoints for fluconazole and Candida revisited: a blueprint for the future of antifungal susceptibility testing.Clin Microbiol Rev. 2006 Apr;19(2):435-47. doi: 10.1128/CMR.19.2.435-447.2006. Clin Microbiol Rev. 2006. PMID: 16614256 Free PMC article. Review.
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
Cited by
-
High-dose fluconazole in combination with amphotericin B is more efficient than monotherapy in murine model of cryptococcosis.Sci Rep. 2017 Jul 5;7(1):4661. doi: 10.1038/s41598-017-04588-7. Sci Rep. 2017. PMID: 28680034 Free PMC article.
-
Efficacy of an abbreviated induction regimen of amphotericin B deoxycholate for cryptococcal meningoencephalitis: 3 days of therapy is equivalent to 14 days.mBio. 2014 Jan 28;5(1):e00725-13. doi: 10.1128/mBio.00725-13. mBio. 2014. PMID: 24473125 Free PMC article.
-
Minimum Inhibitory Concentration Distribution of Fluconazole against Cryptococcus Species and the Fluconazole Exposure Prediction Model.Open Forum Infect Dis. 2019 Oct 1;6(10):ofz369. doi: 10.1093/ofid/ofz369. Open Forum Infect Dis. 2019. PMID: 31420668 Free PMC article.
-
Fluconazole Monotherapy Is a Suboptimal Option for Initial Treatment of Cryptococcal Meningitis Because of Emergence of Resistance.mBio. 2019 Dec 3;10(6):e02575-19. doi: 10.1128/mBio.02575-19. mBio. 2019. PMID: 31796539 Free PMC article.
-
Efficacy and Associated Drug Exposures of Isavuconazole and Fluconazole in an Experimental Model of Coccidioidomycosis.Antimicrob Agents Chemother. 2021 May 18;65(6):e02344-20. doi: 10.1128/AAC.02344-20. Print 2021 May 18. Antimicrob Agents Chemother. 2021. PMID: 33782009 Free PMC article.
References
-
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525–530 - PubMed
-
- Longley N, Muzoora C, Taseera K, Mwesigye J, Rwebembera J, Chakera A, Wall E, Andia I, Jaffar S, Harrison TS. 2008. Dose response effect of high-dose fluconazole for HIV-associated cryptococcal meningitis in southwestern Uganda. Clin. Infect. Dis. 47:1556–1561 - PubMed
-
- Milefchik E, Leal MA, Haubrich R, Bozzette SA, Tilles JG, Leedom JM, McCutchan JA, Larsen RA. 2008. Fluconazole alone or combined with flucytosine for the treatment of AIDS-associated cryptococcal meningitis. Med. Mycol. 46:393–395 - PubMed
-
- Bicanic T, Harrison T, Niepieklo A, Dyakopu N, Meintjes G. 2006. Symptomatic relapse of HIV-associated cryptococcal meningitis after initial fluconazole monotherapy: the role of fluconazole resistance and immune reconstitution. Clin. Infect. Dis. 43:1069–1073 - PubMed
-
- Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts, 3rd ed Approved standard M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

