Performance of pharmacovigilance signal-detection algorithms for the FDA adverse event reporting system
- PMID: 23571771
- PMCID: PMC3857139
- DOI: 10.1038/clpt.2013.24
Performance of pharmacovigilance signal-detection algorithms for the FDA adverse event reporting system
Abstract
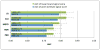
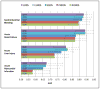
Signal-detection algorithms (SDAs) are recognized as vital tools in pharmacovigilance. However, their performance characteristics are generally unknown. By leveraging a unique gold standard recently made public by the Observational Medical Outcomes Partnership (OMOP) and by conducting a unique systematic evaluation, we provide new insights into the diagnostic potential and characteristics of SDAs that are routinely applied to the US Food and Drug Administration (FDA) Adverse Event Reporting System (AERS). We find that SDAs can attain reasonable predictive accuracy in signaling adverse events. Two performance classes emerge, indicating that the class of approaches that address confounding and masking effects benefits safety surveillance. Our study shows that not all events are equally detectable, suggesting that specific events might be monitored more effectively using other data sources. We provide performance guidelines for several operating scenarios to inform the trade-off between sensitivity and specificity for specific use cases. We also propose an approach and demonstrate its application in identifying optimal signaling thresholds, given specific misclassification tolerances.
Conflict of interest statement
No conflicts to disclose.
Figures

Comment in
-
Advancing the science of pharmacovigilance.Clin Pharmacol Ther. 2013 Jun;93(6):474-5. doi: 10.1038/clpt.2013.60. Clin Pharmacol Ther. 2013. PMID: 23689213
-
Logistic regression in signal detection: another piece added to the puzzle.Clin Pharmacol Ther. 2013 Sep;94(3):312. doi: 10.1038/clpt.2013.107. Epub 2013 May 21. Clin Pharmacol Ther. 2013. PMID: 23695184 No abstract available.
-
Response to "Logistic regression in signal detection: another piece added to the puzzle".Clin Pharmacol Ther. 2013 Sep;94(3):313. doi: 10.1038/clpt.2013.125. Epub 2013 Jun 12. Clin Pharmacol Ther. 2013. PMID: 23756371 No abstract available.
References
-
- [accessed Oct 2012];Adverse Event Reporting System. http://www.fda.gov/cder/aers/default.htm.
-
- Szarfman A, Machado SG, O’Neill RT. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA’s spontaneous reports database. Drug Saf. 2002;25(6):381–392. - PubMed
-
- Szarfman A, Tonning JM, Doraiswamy PM. Pharmacovigilance in the 21st century: new systematic tools for an old problem. Pharmacotherapy. 2004;24(9):1099–1104. - PubMed
-
- Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18(6):427–436. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

