Alterations in metabolic pathways and networks in Alzheimer's disease
- PMID: 23571809
- PMCID: PMC3641405
- DOI: 10.1038/tp.2013.18
Alterations in metabolic pathways and networks in Alzheimer's disease
Abstract
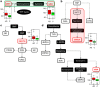
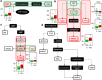
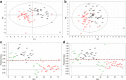
The pathogenic mechanisms of Alzheimer's disease (AD) remain largely unknown and clinical trials have not demonstrated significant benefit. Biochemical characterization of AD and its prodromal phase may provide new diagnostic and therapeutic insights. We used targeted metabolomics platform to profile cerebrospinal fluid (CSF) from AD (n=40), mild cognitive impairment (MCI, n=36) and control (n=38) subjects; univariate and multivariate analyses to define between-group differences; and partial least square-discriminant analysis models to classify diagnostic groups using CSF metabolomic profiles. A partial correlation network was built to link metabolic markers, protein markers and disease severity. AD subjects had elevated methionine (MET), 5-hydroxyindoleacetic acid (5-HIAA), vanillylmandelic acid, xanthosine and glutathione versus controls. MCI subjects had elevated 5-HIAA, MET, hypoxanthine and other metabolites versus controls. Metabolite ratios revealed changes within tryptophan, MET and purine pathways. Initial pathway analyses identified steps in several pathways that appear altered in AD and MCI. A partial correlation network showed total tau most directly related to norepinephrine and purine pathways; amyloid-β (Ab42) was related directly to an unidentified metabolite and indirectly to 5-HIAA and MET. These findings indicate that MCI and AD are associated with an overlapping pattern of perturbations in tryptophan, tyrosine, MET and purine pathways, and suggest that profound biochemical alterations are linked to abnormal Ab42 and tau metabolism. Metabolomics provides powerful tools to map interlinked biochemical pathway perturbations and study AD as a disease of network failure.
Figures

References
-
- Kaddurah-Daouk R, Krishnan KR. Metabolomics: a global biochemical approach to the study of central nervous system diseases. Neuropsychopharmacology. 2009;34:173–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

