DFT studies of the conversion of four mesylate esters during reaction with ammonia
- PMID: 23571822
- PMCID: PMC3713272
- DOI: 10.1007/s00894-013-1835-7
DFT studies of the conversion of four mesylate esters during reaction with ammonia
Abstract
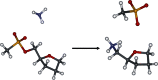
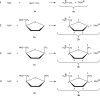
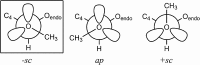
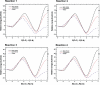
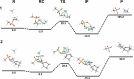
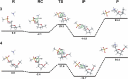
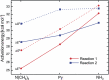
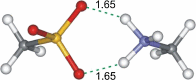
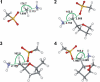
The energetics of the Menshutkin-like reaction between four mesylate derivatives and ammonia have been computed using B3LYP functional with the 6-31+G** basis set. Additionally, MPW1K/6-31+G** level calculations were carried out to estimate activation barrier heights in the gas phase. Solvent effect corrections were computed using PCM/B3LYP/6-31+G** level. The conversion of the reactant complexes into ion pairs is accompanied by a strong energy decrease in the gas phase and in all solvents. The ion pairs are stabilized with two strong hydrogen bonds in the gas phase. The bifurcation at C2 causes a significant activation barrier increase. Also, bifurcation at C5 leads to noticeable barrier height differentiation. Both B3LYP/6-31+G** and MPW1K/6-31+G** activation barriers suggest the reaction 2 (2a + NH3) to be the fastest in the gas phase. The reaction 4 is the slowest one in all environments.
Figures

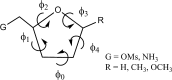
Similar articles
-
The conformational behavior, geometry and energy parameters of Menshutkin-like reaction of O-isopropylidene-protected glycofuranoid mesylates in view of DFT calculations.J Mol Graph Model. 2014 Jul;52:91-102. doi: 10.1016/j.jmgm.2014.06.009. Epub 2014 Jul 1. J Mol Graph Model. 2014. PMID: 25023664
-
DFT studies of the formation of furanoid derivatives of ammonium chlorides.J Mol Graph Model. 2015 Mar;56:74-83. doi: 10.1016/j.jmgm.2014.12.004. Epub 2014 Dec 25. J Mol Graph Model. 2015. PMID: 25562663
-
Density functional theory-based prediction of the formation constants of complexes of ammonia in aqueous solution: indications of the role of relativistic effects in the solution chemistry of gold(I).Inorg Chem. 2005 Oct 3;44(20):7175-83. doi: 10.1021/ic050471s. Inorg Chem. 2005. PMID: 16180881
-
o-Quinone methide as alkylating agent of nitrogen, oxygen, and sulfur nucleophiles. The role of H-bonding and solvent effects on the reactivity through a DFT computational study.J Am Chem Soc. 2001 Aug 29;123(34):8366-77. doi: 10.1021/ja010433h. J Am Chem Soc. 2001. PMID: 11516286
-
A molecular perspective on lithium-ammonia solutions.Angew Chem Int Ed Engl. 2009;48(44):8198-232. doi: 10.1002/anie.200900373. Angew Chem Int Ed Engl. 2009. PMID: 19821473 Review.
Cited by
-
Theoretical investigations on the synthesis mechanism of cyanuric acid from NH₃ and CO₂.J Mol Model. 2013 Nov;19(11):5037-43. doi: 10.1007/s00894-013-2003-9. J Mol Model. 2013. PMID: 24077837
-
Perfluorinated compounds exposure and atherogenic risk characteristics in a high-fat diet condition: In vitro/in vivo models and population panel study.PNAS Nexus. 2025 May 9;4(5):pgaf153. doi: 10.1093/pnasnexus/pgaf153. eCollection 2025 May. PNAS Nexus. 2025. PMID: 40386678 Free PMC article.
-
DFT studies of conversion of methyl chloride and three substituted chloromethyl tetrahydrofuran derivatives during reaction with trimethylamine.J Mol Model. 2013 Oct;19(10):4403-17. doi: 10.1007/s00894-013-1940-7. Epub 2013 Aug 6. J Mol Model. 2013. PMID: 23918221 Free PMC article.
References
-
- Menschutkin N. Z Physik Chem. 1890;5:589–601.
-
- Fischer E, Raske K. Chem Ber. 1910;43:1750–1753. doi: 10.1002/cber.19100430291. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

