Spatiotemporal regulation of meiotic recombination by Liaisonin
- PMID: 23572041
- PMCID: PMC3639241
- DOI: 10.4161/bioa.23966
Spatiotemporal regulation of meiotic recombination by Liaisonin
Abstract
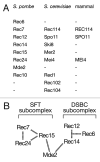
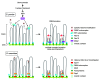
Sexual reproduction involves diversification of genetic information in successive generations. Meiotic recombination, which substantially contributes to the increase in genetic diversity, is initiated by programmed DNA double-strand breaks (DSBs) catalyzed by the evolutionarily conserved Spo11 protein. Spo11 requires additional partner proteins for its DNA cleavage reaction. DSBs are preferentially introduced at defined chromosomal sites called "recombination hotspots." Recent studies have revealed that meiotically established higher-order chromosome structures, such as chromosome axes and loops, are also crucial in the control of DSB formation. Most of the DSB sites are located within chromatin loop regions, while many of the proteins involved in DSB formation reside on chromosomal axes. Hence, DSB proteins and DSB sites seem to be distantly located. To resolve this paradox, we conducted comprehensive proteomics and ChIP-chip analyses on Spo11 partners in Schizosaccharomyces pombe, in combination with mutant studies. We identified two distinct DSB complexes, the "DSBC (DSB Catalytic core)" and "SFT (Seven-Fifteen-Twenty four; Rec7-Rec15-Rec24)" subcomplexes. The DSBC subcomplex contains Spo11 and functions as the catalytic core for the DNA cleavage reaction. The SFT subcomplex is assumed to execute regulatory functions. To activate the DSBC subcomplex, the SFT subcomplex tethers hotspots to axes via its interaction with Mde2, which can interact with proteins in both DSBC and SFT subcomplexes. Thus, Mde2 is likely to bridge these two subcomplexes, forming a "tethered loop-axis complex." It should be noted that Mde2 expression is strictly regulated by S phase checkpoint monitoring of the completion of DNA replication. From these observations, we proposed that Mde2 is a central coupler for meiotic recombination initiation to establish a tethered loop-axis complex in liaison with the S phase checkpoint.
Keywords: DNA double-strand break formation; Meiotic recombination; S phase checkpoint; Spo11; higher-order chromosome structure.
Figures
Comment on
- Miyoshi T, Ito M, Kugou K, Yamada S, Furuichi M, Oda A, et al. A central coupler for recombination initiation linking chromosome architecture to S phase checkpoint. Mol Cell. 2012;47:722–33. doi: 10.1016/j.molcel.2012.06.023.
Similar articles
-
A central coupler for recombination initiation linking chromosome architecture to S phase checkpoint.Mol Cell. 2012 Sep 14;47(5):722-33. doi: 10.1016/j.molcel.2012.06.023. Epub 2012 Jul 26. Mol Cell. 2012. PMID: 22841486
-
The histone variant H2A.Z promotes initiation of meiotic recombination in fission yeast.Nucleic Acids Res. 2018 Jan 25;46(2):609-620. doi: 10.1093/nar/gkx1110. Nucleic Acids Res. 2018. PMID: 29145618 Free PMC article.
-
Functional interactions of Rec24, the fission yeast ortholog of mouse Mei4, with the meiotic recombination-initiation complex.J Cell Sci. 2011 Apr 15;124(Pt 8):1328-38. doi: 10.1242/jcs.079194. Epub 2011 Mar 23. J Cell Sci. 2011. PMID: 21429938 Free PMC article.
-
Initiation of meiotic recombination in chromatin structure.J Biochem. 2013 Aug;154(2):107-14. doi: 10.1093/jb/mvt054. Epub 2013 Jun 8. J Biochem. 2013. PMID: 23750029 Review.
-
The conserved histone variant H2A.Z illuminates meiotic recombination initiation.Curr Genet. 2018 Oct;64(5):1015-1019. doi: 10.1007/s00294-018-0825-9. Epub 2018 Mar 16. Curr Genet. 2018. PMID: 29549582 Review.
Cited by
-
Phospho-Regulation of Meiotic Prophase.Front Cell Dev Biol. 2021 Apr 13;9:667073. doi: 10.3389/fcell.2021.667073. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33928091 Free PMC article. Review.
-
Mechanism and regulation of meiotic recombination initiation.Cold Spring Harb Perspect Biol. 2014 Oct 16;7(1):a016634. doi: 10.1101/cshperspect.a016634. Cold Spring Harb Perspect Biol. 2014. PMID: 25324213 Free PMC article. Review.
-
Asy2/Mer2: an evolutionarily conserved mediator of meiotic recombination, pairing, and global chromosome compaction.Genes Dev. 2017 Sep 15;31(18):1880-1893. doi: 10.1101/gad.304543.117. Epub 2017 Oct 11. Genes Dev. 2017. PMID: 29021238 Free PMC article.
-
Recombination, Pairing, and Synapsis of Homologs during Meiosis.Cold Spring Harb Perspect Biol. 2015 May 18;7(6):a016626. doi: 10.1101/cshperspect.a016626. Cold Spring Harb Perspect Biol. 2015. PMID: 25986558 Free PMC article. Review.
-
Intragenic meiotic recombination in Schizosaccharomyces pombe is sensitive to environmental temperature changes.Chromosome Res. 2020 Jun;28(2):195-207. doi: 10.1007/s10577-020-09632-3. Epub 2020 Apr 17. Chromosome Res. 2020. PMID: 32303869 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
