Protein kinase C and acute respiratory distress syndrome
- PMID: 23572089
- PMCID: PMC3712519
- DOI: 10.1097/SHK.0b013e318294f85a
Protein kinase C and acute respiratory distress syndrome
Abstract
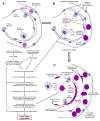
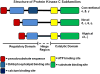
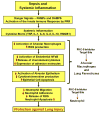
The acute respiratory distress syndrome (ARDS) is a major public health problem and a leading source of morbidity in intensive care units. Lung tissue in patients with ARDS is characterized by inflammation, with exuberant neutrophil infiltration, activation, and degranulation that is thought to initiate tissue injury through the release of proteases and oxygen radicals. Treatment of ARDS is supportive primarily because the underlying pathophysiology is poorly understood. This gap in knowledge must be addressed to identify urgently needed therapies. Recent research efforts in anti-inflammatory drug development have focused on identifying common control points in multiple signaling pathways. The protein kinase C (PKC) serine-threonine kinases are master regulators of proinflammatory signaling hubs, making them attractive therapeutic targets. Pharmacological inhibition of broad-spectrum PKC activity and, more importantly, of specific PKC isoforms (as well as deletion of PKCs in mice) exerts protective effects in various experimental models of lung injury. Furthermore, PKC isoforms have been implicated in inflammatory processes that may be involved in the pathophysiologic changes that result in ARDS, including activation of innate immune and endothelial cells, neutrophil trafficking to the lung, regulation of alveolar epithelial barrier functions, and control of neutrophil proinflammatory and prosurvival signaling. This review focuses on the mechanistic involvement of PKC isoforms in the pathogenesis of ARDS and highlights the potential of developing new therapeutic paradigms based on the selective inhibition (or activation) of specific PKC isoforms.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
References
-
- Ashbaugh D, Bigelow D, Petty T, Levine B. Acute respiratory distress in adults. Lancet. 1967;12:319–323. - PubMed
-
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. American Journal of Respiratory and Critical Care Medicine. 1994;149:818–824. - PubMed
-
- ARDS Definition Task Force. Ranieri V, Rubenfeld G, Thompson B, Ferguson N, Caldwell E, Fan E, Camporota L, Slutsky A. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. - PubMed
-
- Thille AW, Esteban A, Fernandez-Segoviano P, Rodriguez JM, Aramburu JA, Penuelas O, Cortes-Puch I, Cardinal-Fernandez P, Lorente JA, Frutos-Vivar F. Comparison of the Berlin Definition for Acute Respiratory Distress Syndrome with Autopsy. American journal of respiratory and critical care medicine January 31, 2013. 2013 doi: 10.1164/rccm.201211-1981OC. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

