Potential for therapeutic manipulation of the UPR in disease
- PMID: 23572207
- PMCID: PMC3641308
- DOI: 10.1007/s00281-013-0370-z
Potential for therapeutic manipulation of the UPR in disease
Abstract
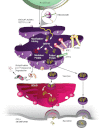
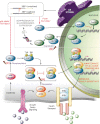
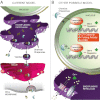
Increased endoplasmic reticulum (ER) stress and the activated unfolded protein response (UPR) signaling associated with it play key roles in physiological processes as well as under pathological conditions. The UPR normally protects cells and re-establishes cellular homeostasis, but prolonged UPR activation can lead to the development of various pathologies. These features make the UPR signaling pathway an attractive target for the treatment of diseases whose pathogenesis is characterized by chronic activation of this pathway. Here, we focus on the molecular signaling pathways of the UPR and suggest possible ways to target this response for therapeutic purposes.
Figures

Similar articles
-
Proteostasis in endoplasmic reticulum--new mechanisms in kidney disease.Nat Rev Nephrol. 2014 Jul;10(7):369-78. doi: 10.1038/nrneph.2014.67. Epub 2014 Apr 22. Nat Rev Nephrol. 2014. PMID: 24752014 Review.
-
The Emerging Role of Electrophiles as a Key Regulator for Endoplasmic Reticulum (ER) Stress.Int J Mol Sci. 2019 Apr 10;20(7):1783. doi: 10.3390/ijms20071783. Int J Mol Sci. 2019. PMID: 30974903 Free PMC article. Review.
-
The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies.Oncogene. 2013 Feb 14;32(7):805-18. doi: 10.1038/onc.2012.130. Epub 2012 Apr 16. Oncogene. 2013. PMID: 22508478 Free PMC article. Review.
-
Control of the Unfolded Protein Response in Health and Disease.SLAS Discov. 2017 Aug;22(7):787-800. doi: 10.1177/2472555217701685. Epub 2017 Apr 28. SLAS Discov. 2017. PMID: 28453376 Review.
-
Unfolded Protein Response Pathways in Neurodegenerative Diseases.J Mol Neurosci. 2015 Dec;57(4):529-37. doi: 10.1007/s12031-015-0633-3. Epub 2015 Aug 25. J Mol Neurosci. 2015. PMID: 26304853 Review.
Cited by
-
Interactions of melatonin with various signaling pathways: implications for cancer therapy.Cancer Cell Int. 2022 Dec 29;22(1):420. doi: 10.1186/s12935-022-02825-2. Cancer Cell Int. 2022. PMID: 36581900 Free PMC article. Review.
-
The regulatory subunits of PI3K, p85α and p85β, differentially affect BRD7-mediated regulation of insulin signaling.J Mol Cell Biol. 2022 Jan 29;13(12):889-901. doi: 10.1093/jmcb/mjab073. J Mol Cell Biol. 2022. PMID: 34751372 Free PMC article.
-
Renaissance of leptin for obesity therapy.Diabetologia. 2016 May;59(5):920-7. doi: 10.1007/s00125-016-3906-7. Epub 2016 Mar 16. Diabetologia. 2016. PMID: 26983921 Review.
-
The unfolded protein response and gastrointestinal disease.Semin Immunopathol. 2013 May;35(3):307-19. doi: 10.1007/s00281-013-0377-5. Epub 2013 Apr 16. Semin Immunopathol. 2013. PMID: 23588234 Free PMC article. Review.
-
Double bond configuration of palmitoleate is critical for atheroprotection.Mol Metab. 2019 Oct;28:58-72. doi: 10.1016/j.molmet.2019.08.004. Epub 2019 Aug 7. Mol Metab. 2019. PMID: 31422082 Free PMC article.
References
-
- Shibata Y, Voeltz GK, Rapoport TA. Rough sheets and smooth tubules. Cell. 2006;126(3):435–439. - PubMed
-
- Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86(4):1133–1149. - PubMed
-
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

