Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections
- PMID: 23572228
- PMCID: PMC6296322
- DOI: 10.1093/jac/dkt111
Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections
Abstract
Objectives: Respiratory syncytial virus (RSV) infections are well recognized as a significant cause of morbidity and mortality in allogeneic haematopoietic stem cell transplant (allo-HSCT) recipients. We evaluated the spectrum of clinical manifestations, management (including ribavirin-based antiviral therapy) and outcomes of RSV infections and determined the risk factors associated with RSV lower respiratory tract infection (LRTI) and all-cause mortality.
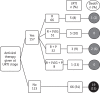
Methods: In this retrospective study, we analysed clinical data from all laboratory-confirmed RSV infections in allo-HSCT recipients (n = 280) who presented at our institution from January 1996 to May 2009.
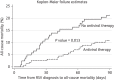
Results: Of the 280 patients, 80 (29%) developed LRTI within 20 days (median 1 day, range 0-19 days) and 44 (16%) died within 90 days (median 26 days, range 1-82 days) from RSV diagnosis. Multivariable logistic regression analyses identified several significant risk factors associated with RSV LRTI and all-cause mortality, including age, male sex, neutropenia, lymphocytopenia and lack of ribavirin-based antiviral therapy at the upper respiratory tract infection (URTI) stage. Aerosolized ribavirin-based therapy at the URTI stage was the single most significant factor in reducing the risk of RSV LRTI (83%), all-cause mortality (57%) and RSV-associated mortality (87%) in these patients (P < 0.05), irrespective of the year of RSV diagnosis.
Conclusions: Our results demonstrate that RSV infections are a significant cause of morbidity and mortality in high-risk allo-HSCT recipients and ribavirin-based antiviral therapy at the URTI stage had a positive impact on both outcomes in this vulnerable population with multiple risk factors.
Keywords: RSV; immunocompromised; pneumonia; stem cell transplantation.
Figures

References
-
- Shah JN, Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood. 2011;117:2755–63. - PubMed
-
- Whimbey E, Champlin RE, Couch RB, et al. Community respiratory virus infections among hospitalized adult bone marrow transplant recipients. Clin Infect Dis. 1996;22:778–82. - PubMed
-
- Khanna N, Widmer AF, Decker M, et al. Respiratory syncytial virus infection in patients with hematological diseases: single-center study and review of the literature. Clin Infect Dis. 2008;46:402–12. - PubMed
-
- Raboni SM, Nogueira MB, Tsuchiya LRV, et al. Respiratory tract viral infections in bone marrow transplant patients. Transplantation. 2003;76:142–6. - PubMed
-
- Martino R, Porras RP, Rabella N, et al. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biol Blood Marrow Transplant. 2005;11:781–96. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

