Telomerase-dependent 3' G-strand overhang maintenance facilitates GTBP1-mediated telomere protection from misplaced homologous recombination
- PMID: 23572544
- PMCID: PMC3663271
- DOI: 10.1105/tpc.112.107573
Telomerase-dependent 3' G-strand overhang maintenance facilitates GTBP1-mediated telomere protection from misplaced homologous recombination
Abstract
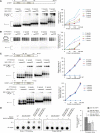
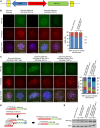
At the 3'-end of telomeres, single-stranded G-overhang telomeric repeats form a stable T-loop. Many studies have focused on the mechanisms that generate and regulate the length of telomere 3' G-strand overhangs, but the roles of G-strand overhang length control in proper T-loop formation and end protection remain unclear. Here, we examined functional relationships between the single-stranded telomere binding protein GTBP1 and G-strand overhang lengths maintained by telomerase in tobacco (Nicotiana tabacum). In tobacco plants, telomerase reverse transcriptase subunit (TERT) repression severely worsened the GTBP1 knockdown phenotypes, which were formally characterized as an outcome of telomere destabilization. TERT downregulation shortened the telomere 3' G-overhangs and increased telomere recombinational aberrations in GTBP1-suppressed plants. Correlatively, GTBP1-mediated inhibition of single-strand invasion into the double-strand telomeric sequences was impaired due to shorter single-stranded telomeres. Moreover, TERT/GTBP1 double knockdown amplified misplaced homologous recombination of G-strand overhangs into intertelomeric regions. Thus, proper G-overhang length maintenance is required to protect telomeres against intertelomeric recombination, which is achieved by the balanced functions of GTBP1 and telomerase activity.
Figures


Similar articles
-
Tobacco GTBP1, a homolog of human heterogeneous nuclear ribonucleoprotein, protects telomeres from aberrant homologous recombination.Plant Cell. 2010 Aug;22(8):2781-95. doi: 10.1105/tpc.110.076778. Epub 2010 Aug 26. Plant Cell. 2010. PMID: 20798328 Free PMC article.
-
Erosion of telomeric single-stranded overhang in patients with aplastic anaemia carrying telomerase complex mutations.Eur J Clin Invest. 2009 Nov;39(11):1025-32. doi: 10.1111/j.1365-2362.2009.02209.x. Epub 2009 Aug 11. Eur J Clin Invest. 2009. PMID: 19674077 Free PMC article.
-
Telomeric overhang length determines structural dynamics and accessibility to telomerase and ALT-associated proteins.Structure. 2014 Jun 10;22(6):842-53. doi: 10.1016/j.str.2014.03.013. Epub 2014 May 15. Structure. 2014. PMID: 24836024 Free PMC article.
-
Telomere C-Strand Fill-In Machinery: New Insights into the Human CST-DNA Polymerase Alpha-Primase Structures and Functions.Subcell Biochem. 2024;104:73-100. doi: 10.1007/978-3-031-58843-3_5. Subcell Biochem. 2024. PMID: 38963484 Review.
-
Telomeric and extra-telomeric roles for telomerase and the telomere-binding proteins.Nat Rev Cancer. 2011 Mar;11(3):161-76. doi: 10.1038/nrc3025. Nat Rev Cancer. 2011. PMID: 21346783 Review.
Cited by
-
Telomere- and Telomerase-Associated Proteins and Their Functions in the Plant Cell.Front Plant Sci. 2016 Jun 28;7:851. doi: 10.3389/fpls.2016.00851. eCollection 2016. Front Plant Sci. 2016. PMID: 27446102 Free PMC article. Review.
-
Telomerase activity is required for the telomere G-overhang structure in Trypanosoma brucei.Sci Rep. 2017 Nov 22;7(1):15983. doi: 10.1038/s41598-017-16182-y. Sci Rep. 2017. PMID: 29167542 Free PMC article.
-
TRF1 participates in chromosome end protection by averting TRF2-dependent telomeric R loops.Nat Struct Mol Biol. 2018 Feb;25(2):147-153. doi: 10.1038/s41594-017-0021-5. Epub 2018 Jan 22. Nat Struct Mol Biol. 2018. PMID: 29358759 Free PMC article.
-
Revisiting regulatory roles of replication protein A in plant DNA metabolism.Planta. 2021 May 28;253(6):130. doi: 10.1007/s00425-021-03641-0. Planta. 2021. PMID: 34047822 Review.
References
-
- Amiard S., et al. (2007). A topological mechanism for TRF2-enhanced strand invasion. Nat. Struct. Mol. Biol. 14: 147–154 - PubMed
-
- Bailey S.M., Cornforth M.N., Kurimasa A., Chen D.J., Goodwin E.H. (2001). Strand-specific postreplicative processing of mammalian telomeres. Science 293: 2462–2465 - PubMed
-
- Blackburn E.H. (1991). Structure and function of telomeres. Nature 350: 569–573 - PubMed
-
- Bonetti D., Martina M., Clerici M., Lucchini G., Longhese M.P. (2009). Multiple pathways regulate 3′ overhang generation at S. cerevisiae telomeres. Mol. Cell 35: 70–81 - PubMed
-
- Cesare A.J., Reddel R.R. (2010). Alternative lengthening of telomeres: Models, mechanisms and implications. Nat. Rev. Genet. 11: 319–330 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

