LOXL4 is induced by transforming growth factor β1 through Smad and JunB/Fra2 and contributes to vascular matrix remodeling
- PMID: 23572561
- PMCID: PMC3700097
- DOI: 10.1128/MCB.00036-13
LOXL4 is induced by transforming growth factor β1 through Smad and JunB/Fra2 and contributes to vascular matrix remodeling
Abstract
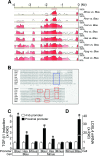
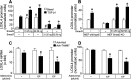
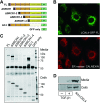
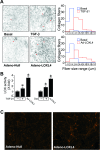
Transforming growth factor β1 (TGF-β1) is a pleiotropic factor involved in the regulation of extracellular matrix (ECM) synthesis and remodeling. In search for novel genes mediating the action of TGF-β1 on vascular ECM, we identified the member of the lysyl oxidase family of matrix-remodeling enzymes, lysyl oxidase-like 4 (LOXL4), as a direct target of TGF-β1 in aortic endothelial cells, and we dissected the molecular mechanism of its induction. Deletion mapping and mutagenesis analysis of the LOXL4 promoter demonstrated the absolute requirement of a distal enhancer containing an activator protein 1 (AP-1) site and a Smad binding element for TGF-β1 to induce LOXL4 expression. Functional cooperation between Smad proteins and the AP-1 complex composed of JunB/Fra2 accounted for the action of TGF-β1, which involved the extracellular signal-regulated kinase (ERK)-dependent phosphorylation of Fra2. We furthermore provide evidence that LOXL4 was extracellularly secreted and significantly contributed to ECM deposition and assembly. These results suggest that TGF-β1-dependent expression of LOXL4 plays a role in vascular ECM homeostasis, contributing to vascular processes associated with ECM remodeling and fibrosis.
Figures





References
-
- Wu MY, Hill CS. 2009. Tgf-beta superfamily signaling in embryonic development and homeostasis. Dev. Cell 16:329–343 - PubMed
-
- Attisano L, Wrana JL. 2002. Signal transduction by the TGF-beta superfamily. Science 296:1646–1647 - PubMed
-
- Leask A, Abraham DJ. 2004. TGF-beta signaling and the fibrotic response. FASEB J. 18:816–827 - PubMed
-
- Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. 2007. TGF-beta signaling in vascular fibrosis. Cardiovasc. Res. 74:196–206 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
