Transposon mutagenesis identifies genes essential for Plasmodium falciparum gametocytogenesis
- PMID: 23572579
- PMCID: PMC3645567
- DOI: 10.1073/pnas.1217712110
Transposon mutagenesis identifies genes essential for Plasmodium falciparum gametocytogenesis
Abstract
Gametocytes are essential for Plasmodium transmission, but little is known about the mechanisms that lead to their formation. Using piggyBac transposon-mediated insertional mutagenesis, we screened for parasites that no longer form mature gametocytes, which led to the isolation of 29 clones (insertional gametocyte-deficient mutants) that fail to form mature gametocytes. Additional analysis revealed 16 genes putatively responsible for the loss of gametocytogenesis, none of which has been previously implicated in gametocytogenesis. Transcriptional profiling and detection of an early stage gametocyte antigen determined that a subset of these mutants arrests development at stage I or in early stage II gametocytes, likely representing genes involved in gametocyte maturation. The remaining mutants seem to arrest before formation of stage I gametocytes and may represent genes involved in commitment to the gametocyte lineage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

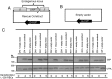
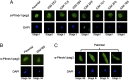

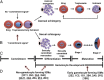

Similar articles
-
Pyp25α is required for male gametocyte exflagellation.Pathog Dis. 2022 Nov 12;80(1):ftac043. doi: 10.1093/femspd/ftac043. Pathog Dis. 2022. PMID: 36316012
-
Phenotypic Screens Identify Genetic Factors Associated with Gametocyte Development in the Human Malaria Parasite Plasmodium falciparum.Microbiol Spectr. 2023 Jun 15;11(3):e0416422. doi: 10.1128/spectrum.04164-22. Epub 2023 May 8. Microbiol Spectr. 2023. PMID: 37154686 Free PMC article.
-
A 39-Amino-Acid C-Terminal Truncation of GDV1 Disrupts Sexual Commitment in Plasmodium falciparum.mSphere. 2021 May 19;6(3):e01093-20. doi: 10.1128/mSphere.01093-20. mSphere. 2021. PMID: 34011691 Free PMC article.
-
Functional genomics of Plasmodium falciparum through transposon-mediated mutagenesis.Cell Microbiol. 2006 Oct;8(10):1529-36. doi: 10.1111/j.1462-5822.2006.00776.x. Cell Microbiol. 2006. PMID: 16984409 Review.
-
Plasmodium falciparum gametocytes: still many secrets of a hidden life.Mol Microbiol. 2007 Oct;66(2):291-302. doi: 10.1111/j.1365-2958.2007.05904.x. Epub 2007 Sep 3. Mol Microbiol. 2007. PMID: 17784927 Review.
Cited by
-
Regulation of Sexual Commitment and Gametocytogenesis in Malaria Parasites.Annu Rev Microbiol. 2018 Sep 8;72:501-519. doi: 10.1146/annurev-micro-090817-062712. Epub 2018 Jul 5. Annu Rev Microbiol. 2018. PMID: 29975590 Free PMC article. Review.
-
Transcriptome sequencing and analysis of Plasmodium gallinaceum reveals polymorphisms and selection on the apical membrane antigen-1.Malar J. 2014 Sep 26;13:382. doi: 10.1186/1475-2875-13-382. Malar J. 2014. PMID: 25261185 Free PMC article.
-
Assembling the components of the quorum sensing pathway in African trypanosomes.Mol Microbiol. 2015 Apr;96(2):220-32. doi: 10.1111/mmi.12949. Epub 2015 Mar 4. Mol Microbiol. 2015. PMID: 25630552 Free PMC article. Review.
-
Declines in prevalence alter the optimal level of sexual investment for the malaria parasite Plasmodium falciparum.Proc Natl Acad Sci U S A. 2022 Jul 26;119(30):e2122165119. doi: 10.1073/pnas.2122165119. Epub 2022 Jul 22. Proc Natl Acad Sci U S A. 2022. PMID: 35867831 Free PMC article.
-
Systems analysis of host-parasite interactions.Wiley Interdiscip Rev Syst Biol Med. 2015 Nov-Dec;7(6):381-400. doi: 10.1002/wsbm.1311. Epub 2015 Aug 26. Wiley Interdiscip Rev Syst Biol Med. 2015. PMID: 26306749 Free PMC article. Review.
References
-
- Inselburg J. Gametocyte formation by the progeny of single Plasmodium falciparum schizonts. J Parasitol. 1983;69(3):584–591. - PubMed
-
- Bruce MC, Alano P, Duthie S, Carter R. Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology. 1990;100(Pt 2):191–200. - PubMed
-
- Silvestrini F, Alano P, Williams JL. Commitment to the production of male and female gametocytes in the human malaria parasite Plasmodium falciparum. Parasitology. 2000;121(Pt 5):465–471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

