Genetic transformation of Indian isolate of Lemna minor mediated by Agrobacterium tumefaciens and recovery of transgenic plants
- PMID: 23573002
- PMCID: PMC3550542
- DOI: 10.1007/s12298-011-0059-5
Genetic transformation of Indian isolate of Lemna minor mediated by Agrobacterium tumefaciens and recovery of transgenic plants
Abstract
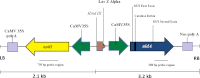
Transgenic plants of an Indian isolate of Lemna minor have been developed for the first time using Agrobacterium tumefaciens and hard nodular cell masses 'nodular calli' developed on the BAP - pretreated daughter frond explants in B5 medium containing sucrose (1.0 %) with 2,4-D (5.0 μM) and 2-iP (50.0 μM) or 2,4-D (50.0 μM) and TDZ (5.0 μM) under light conditions. These calli were co-cultured with A. tumefaciens strain EHA105 harboring a binary vector that contained genes for β-glucuronidase with intron and neomycin phosphortransferase. Transformed cells selected on kanamycin selection medium were regenerated into fronds whose transgenic nature was confirmed by histochemical assay for GUS activity, PCR analysis and Southern hybridization. The frequency of transformation obtained was 3.8 % and a period of 11-13 weeks was required from initiation of cultures from explants to fully grown transgenic fronds. The pretreatment of daughter fronds with BAP, use of non-ionic surfactant, presence of acetosyringone in co-cultivation medium, co-culture duration of 3 d and 16 h photoperiod during culture were found crucial for callus induction, frond regeneration and transformation of L. minor. This transformation system can be used for the production of pharmaceutically important protein and in bioremediation.
Keywords: Agrobacterium-mediated transformation; Lemna minor; Pharmaceutical proteins; Tissue culture; Transgenic plants.
Figures



Similar articles
-
Agrobacterium-Mediated Genetic Transformation of Taiwanese Isolates of Lemna aequinoctialis.Plants (Basel). 2021 Jul 30;10(8):1576. doi: 10.3390/plants10081576. Plants (Basel). 2021. PMID: 34451621 Free PMC article.
-
Agrobacterium-mediated genetic transformation and development of herbicide-resistant sugarcane (Saccharum species hybrids) using axillary buds.Plant Cell Rep. 2004 Sep;23(3):134-43. doi: 10.1007/s00299-004-0794-y. Epub 2004 May 5. Plant Cell Rep. 2004. PMID: 15133712
-
Efficient genetic transformation of Withania coagulans (Stocks) Dunal mediated by Agrobacterium tumefaciens from leaf explants of in vitro multiple shoot culture.Protoplasma. 2013 Apr;250(2):451-8. doi: 10.1007/s00709-012-0428-0. Epub 2012 Jul 6. Protoplasma. 2013. PMID: 22766977
-
Agrobacterium tumefaciens-mediated transformation of eggplant (Solanum melongena L.) using root explants.Plant Cell Rep. 2003 Feb;21(6):549-54. doi: 10.1007/s00299-002-0546-9. Epub 2003 Jan 8. Plant Cell Rep. 2003. PMID: 12789429
-
Transgenic plants of coffee Coffea canephora from embryogenic callus via Agrobacterium tumefaciens-mediated transformation.Plant Cell Rep. 1999 Dec;19(2):106-110. doi: 10.1007/s002990050719. Plant Cell Rep. 1999. PMID: 30754734
Cited by
-
Agrobacterium-Mediated Genetic Transformation of Taiwanese Isolates of Lemna aequinoctialis.Plants (Basel). 2021 Jul 30;10(8):1576. doi: 10.3390/plants10081576. Plants (Basel). 2021. PMID: 34451621 Free PMC article.
-
An Efficient Agrobacterium-Mediated Transformation Method for Hybrid Poplar 84K (Populus alba × P. glandulosa) Using Calli as Explants.Int J Mol Sci. 2022 Feb 17;23(4):2216. doi: 10.3390/ijms23042216. Int J Mol Sci. 2022. PMID: 35216331 Free PMC article.
-
Duckweed: a potential phytosensor for heavy metals.Plant Cell Rep. 2022 Dec;41(12):2231-2243. doi: 10.1007/s00299-022-02913-7. Epub 2022 Aug 18. Plant Cell Rep. 2022. PMID: 35980444 Review.
-
Frond transformation system mediated by Agrobacterium tumefaciens for Lemna minor.Plant Mol Biol. 2018 Nov;98(4-5):319-331. doi: 10.1007/s11103-018-0778-x. Epub 2018 Oct 8. Plant Mol Biol. 2018. PMID: 30298427
-
Research Progress of a Potential Bioreactor: Duckweed.Biomolecules. 2021 Jan 13;11(1):93. doi: 10.3390/biom11010093. Biomolecules. 2021. PMID: 33450858 Free PMC article. Review.
References
-
- Brunning JL, Kintz BL. Computational hand book of statistics. 2. Glenview: Scott Foresman; 1977.
-
- Cheng JJ, Stomp AM. Growing duckweed to recover nutrients from wastewaters and for production of fuel ethanol and animal feed. Clean. 2009;37:17–26.
LinkOut - more resources
Full Text Sources
Other Literature Sources
