Robust and highly-efficient differentiation of functional monocytic cells from human pluripotent stem cells under serum- and feeder cell-free conditions
- PMID: 23573196
- PMCID: PMC3616072
- DOI: 10.1371/journal.pone.0059243
Robust and highly-efficient differentiation of functional monocytic cells from human pluripotent stem cells under serum- and feeder cell-free conditions
Abstract
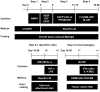
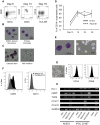
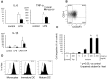
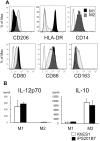
Monocytic lineage cells (monocytes, macrophages and dendritic cells) play important roles in immune responses and are involved in various pathological conditions. The development of monocytic cells from human embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) is of particular interest because it provides an unlimited cell source for clinical application and basic research on disease pathology. Although the methods for monocytic cell differentiation from ESCs/iPSCs using embryonic body or feeder co-culture systems have already been established, these methods depend on the use of xenogeneic materials and, therefore, have a relatively poor-reproducibility. Here, we established a robust and highly-efficient method to differentiate functional monocytic cells from ESCs/iPSCs under serum- and feeder cell-free conditions. This method produced 1.3 × 10(6) ± 0.3 × 10(6) floating monocytes from approximately 30 clusters of ESCs/iPSCs 5-6 times per course of differentiation. Such monocytes could be differentiated into functional macrophages and dendritic cells. This method should be useful for regenerative medicine, disease-specific iPSC studies and drug discovery.
Conflict of interest statement
Figures

Similar articles
-
Efficient derivation and genetic modifications of human pluripotent stem cells on engineered human feeder cell lines.Stem Cells Dev. 2012 Aug 10;21(12):2298-311. doi: 10.1089/scd.2011.0688. Epub 2012 Feb 15. Stem Cells Dev. 2012. PMID: 22225458 Free PMC article.
-
Gingival Fibroblasts as Autologous Feeders for Induced Pluripotent Stem Cells.J Dent Res. 2016 Jan;95(1):110-8. doi: 10.1177/0022034515611602. Epub 2015 Oct 14. J Dent Res. 2016. PMID: 26467419
-
Small molecule mesengenic induction of human induced pluripotent stem cells to generate mesenchymal stem/stromal cells.Stem Cells Transl Med. 2012 Feb;1(2):83-95. doi: 10.5966/sctm.2011-0022. Epub 2012 Feb 7. Stem Cells Transl Med. 2012. PMID: 23197756 Free PMC article.
-
Induced pluripotent stem cells (iPSCs) derived from different cell sources and their potential for regenerative and personalized medicine.Curr Mol Med. 2013 Jun;13(5):792-805. doi: 10.2174/1566524011313050010. Curr Mol Med. 2013. PMID: 23642060 Review.
-
The role of microRNAs in embryonic stem cell and induced pluripotent stem cell differentiation in male germ cells.J Cell Physiol. 2019 Aug;234(8):12278-12289. doi: 10.1002/jcp.27990. Epub 2018 Dec 10. J Cell Physiol. 2019. PMID: 30536380 Review.
Cited by
-
Pluripotent cell models of fanconi anemia identify the early pathological defect in human hemoangiogenic progenitors.Stem Cells Transl Med. 2015 Apr;4(4):333-8. doi: 10.5966/sctm.2013-0172. Epub 2015 Mar 11. Stem Cells Transl Med. 2015. PMID: 25762002 Free PMC article.
-
Restoring regulatory T-cell dysfunction in Alzheimer's disease through ex vivo expansion.Brain Commun. 2020 Jul 20;2(2):fcaa112. doi: 10.1093/braincomms/fcaa112. eCollection 2020. Brain Commun. 2020. PMID: 32954348 Free PMC article.
-
A portable platform for stepwise hematopoiesis from human pluripotent stem cells within PET-reinforced collagen sponges.Int J Hematol. 2016 Dec;104(6):647-660. doi: 10.1007/s12185-016-2088-x. Epub 2016 Sep 6. Int J Hematol. 2016. PMID: 27599982
-
Generation of HIV-Resistant Macrophages from IPSCs by Using Transcriptional Gene Silencing and Promoter-Targeted RNA.Mol Ther Nucleic Acids. 2018 Sep 7;12:793-804. doi: 10.1016/j.omtn.2018.07.017. Epub 2018 Aug 4. Mol Ther Nucleic Acids. 2018. PMID: 30141412 Free PMC article.
-
Pluripotent Stem Cell Model of Nakajo-Nishimura Syndrome Untangles Proinflammatory Pathways Mediated by Oxidative Stress.Stem Cell Reports. 2018 Jun 5;10(6):1835-1850. doi: 10.1016/j.stemcr.2018.04.004. Epub 2018 May 3. Stem Cell Reports. 2018. PMID: 29731430 Free PMC article.
References
-
- Auffray C, Sieweke MH, Geissmann F (2009) Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol 27: 669–692. - PubMed
-
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23: 549–555. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

