New putative chloroplast vesicle transport components and cargo proteins revealed using a bioinformatics approach: an Arabidopsis model
- PMID: 23573218
- PMCID: PMC3613420
- DOI: 10.1371/journal.pone.0059898
New putative chloroplast vesicle transport components and cargo proteins revealed using a bioinformatics approach: an Arabidopsis model
Abstract
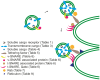
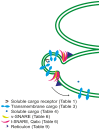
Proteins and lipids are known to be transported to targeted cytosolic compartments in vesicles. A similar system in chloroplasts is suggested to transfer lipids from the inner envelope to the thylakoids. However, little is known about both possible cargo proteins and the proteins required to build a functional vesicle transport system in chloroplasts. A few components have been suggested, but only one (CPSAR1) has a verified location in chloroplast vesicles. This protein is localized in the donor membrane (envelope) and vesicles, but not in the target membrane (thylakoids) suggesting it plays a similar role to a cytosolic homologue, Sar1, in the secretory pathway. Thus, we hypothesized that there may be more similarities, in addition to lipid transport, between the vesicle transport systems in the cytosol and chloroplast, i.e. similar vesicle transport components, possible cargo proteins and receptors. Therefore, using a bioinformatics approach we searched for putative chloroplast components in the model plant Arabidopsis thaliana, corresponding mainly to components of the cytosolic vesicle transport system that may act in coordination with previously proposed COPII chloroplast homologues. We found several additional possible components, supporting the notion of a fully functional vesicle transport system in chloroplasts. Moreover, we found motifs in thylakoid-located proteins similar to those of COPII vesicle cargo proteins, supporting the hypothesis that chloroplast vesicles may transport thylakoid proteins from the envelope to the thylakoid membrane. Several putative cargo proteins are involved in photosynthesis, thus we propose the existence of a novel thylakoid protein pathway that is important for construction and maintenance of the photosynthetic machinery.
Conflict of interest statement
Figures




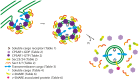

References
-
- Abdallah F, Salamini F, Leister D (2000) A prediction of the size and evolutionary origin of the proteome of chloroplasts of Arabidopsis. Trends Plant Science 5: 141–142. - PubMed
-
- Aronsson H, Jarvis P (2009) The chloroplast protein import apparatus, its components, and their roles. The Chloroplast: 89–123.
-
- Jarvis P, Robinson C (2004) Mechanisms of protein import and routing in chloroplasts. Current Biology 14: R1064–R1077. - PubMed
-
- Robinson C, Thompson SJ, Woolhead C (2001) Multiple pathways used for the targeting of thylakoid proteins in chloroplasts. Traffic 2: 245–251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

