A phase 1 randomized, double blind, placebo controlled rectal safety and acceptability study of tenofovir 1% gel (MTN-007)
- PMID: 23573238
- PMCID: PMC3616022
- DOI: 10.1371/journal.pone.0060147
A phase 1 randomized, double blind, placebo controlled rectal safety and acceptability study of tenofovir 1% gel (MTN-007)
Abstract
Objective: Rectal microbicides are needed to reduce the risk of HIV acquisition associated with unprotected receptive anal intercourse. The MTN-007 study was designed to assess the safety (general and mucosal), adherence, and acceptability of a new reduced glycerin formulation of tenofovir 1% gel.
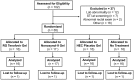
Methods: Participants were randomized 1:1:1:1 to receive the reduced glycerin formulation of tenofovir 1% gel, a hydroxyethyl cellulose placebo gel, a 2% nonoxynol-9 gel, or no treatment. Each gel was administered as a single dose followed by 7 daily doses. Mucosal safety evaluation included histology, fecal calprotectin, epithelial sloughing, cytokine expression (mRNA and protein), microarrays, flow cytometry of mucosal T cell phenotype, and rectal microflora. Acceptability and adherence were determined by computer-administered questionnaires and interactive telephone response, respectively.
Results: Sixty-five participants (45 men and 20 women) were recruited into the study. There were no significant differences between the numbers of ≥ Grade 2 adverse events across the arms of the study. Likelihood of future product use (acceptability) was 87% (reduced glycerin formulation of tenofovir 1% gel), 93% (hydroxyethyl cellulose placebo gel), and 63% (nonoxynol-9 gel). Fecal calprotectin, rectal microflora, and epithelial sloughing did not differ by treatment arms during the study. Suggestive evidence of differences was seen in histology, mucosal gene expression, protein expression, and T cell phenotype. These changes were mostly confined to comparisons between the nonoxynol-9 gel and other study arms.
Conclusions: The reduced glycerin formulation of tenofovir 1% gel was safe and well tolerated rectally and should be advanced to Phase 2 development.
Trial registration: ClinicalTrials.gov NCT01232803.
Conflict of interest statement
Figures

References
-
- Chen SY, Gibson S, Weide D, McFarland W (2003) Unprotected anal intercourse between potentially HIV-serodiscordant men who have sex with men, San Francisco. J Acquir Immune Defic Syndr 33: 166–170. - PubMed
-
- Lane T, Pettifor A, Pascoe S, Fiamma A, Rees H (2006) Heterosexual anal intercourse increases risk of HIV infection among young South African men. AIDS 20: 123–125. - PubMed

