Genomic and structural characterization of Kunitz-type peptide LmKTT-1a highlights diversity and evolution of scorpion potassium channel toxins
- PMID: 23573241
- PMCID: PMC3616063
- DOI: 10.1371/journal.pone.0060201
Genomic and structural characterization of Kunitz-type peptide LmKTT-1a highlights diversity and evolution of scorpion potassium channel toxins
Abstract
Background: Recently, a new subfamily of long-chain toxins with a Kunitz-type fold was found in scorpion venom glands. Functionally, these toxins inhibit protease activity and block potassium channels. However, the genomic organization and three-dimensional (3-D) structure of this kind of scorpion toxin has not been reported.
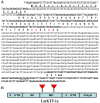
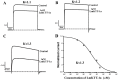
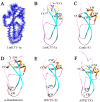
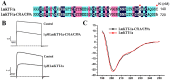
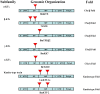
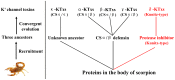
Principal findings: Here, we characterized the genomic organization and 3-D nuclear magnetic resonance structure of the scorpion Kunitz-type toxin, LmKTT-1a, which has a unique cysteine pattern. The LmKTT-1a gene contained three exons, which were interrupted by two introns located in the mature peptide region. Despite little similarity to other Kunitz-type toxins and a unique pattern of disulfide bridges, LmKTT-1a possessed a conserved Kunitz-type structural fold with one α-helix and two β-sheets. Comparison of the genomic organization, 3-D structure, and functional data of known toxins from the α-KTx, β-KTx, γ-KTx, and κ-KTx subfamily suggested that scorpion Kunitz-type potassium channel toxins might have evolved from a new ancestor that is completely different from the common ancestor of scorpion toxins with a CSα/β fold. Thus, these analyses provide evidence of a new scorpion potassium channel toxin subfamily, which we have named δ-KTx.
Conclusions/significance: Our results highlight the genomic, structural, and evolutionary diversity of scorpion potassium channel toxins. These findings may accelerate the design and development of diagnostic and therapeutic peptide agents for human potassium channelopathies.
Conflict of interest statement
Figures

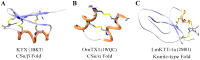

Similar articles
-
Hg1, novel peptide inhibitor specific for Kv1.3 channels from first scorpion Kunitz-type potassium channel toxin family.J Biol Chem. 2012 Apr 20;287(17):13813-21. doi: 10.1074/jbc.M112.343996. Epub 2012 Feb 21. J Biol Chem. 2012. PMID: 22354971 Free PMC article.
-
Functional evolution of scorpion venom peptides with an inhibitor cystine knot fold.Biosci Rep. 2013 Jun 27;33(3):e00047. doi: 10.1042/BSR20130052. Biosci Rep. 2013. PMID: 23721518 Free PMC article.
-
SjAPI-2 is the first member of a new neurotoxin family with Ascaris-type fold and KCNQ1 inhibitory activity.Int J Biol Macromol. 2015 Aug;79:504-10. doi: 10.1016/j.ijbiomac.2015.05.027. Epub 2015 May 23. Int J Biol Macromol. 2015. PMID: 26014142
-
Diverse Structural Features of Potassium Channels Characterized by Scorpion Toxins as Molecular Probes.Molecules. 2019 May 29;24(11):2045. doi: 10.3390/molecules24112045. Molecules. 2019. PMID: 31146335 Free PMC article. Review.
-
The Kv1.3 K+ channel in the immune system and its "precision pharmacology" using peptide toxins.Biol Futur. 2021 Mar;72(1):75-83. doi: 10.1007/s42977-021-00071-7. Epub 2021 Feb 6. Biol Futur. 2021. PMID: 34554500 Review.
Cited by
-
Characterization of Kunitz-Domain Anticoagulation Peptides Derived from Acinetobacter baumannii Exotoxin Protein F6W77.Toxins (Basel). 2024 Oct 21;16(10):450. doi: 10.3390/toxins16100450. Toxins (Basel). 2024. PMID: 39453226 Free PMC article.
-
A Deeper Examination of Thorellius atrox Scorpion Venom Components with Omic Techonologies.Toxins (Basel). 2017 Dec 12;9(12):399. doi: 10.3390/toxins9120399. Toxins (Basel). 2017. PMID: 29231872 Free PMC article.
-
The proteotranscriptomic characterization of venom in the white seafan Eunicella singularis elucidates the evolution of Octocorallia arsenal.Open Biol. 2025 Mar;15(3):250015. doi: 10.1098/rsob.250015. Epub 2025 Mar 12. Open Biol. 2025. PMID: 40068811 Free PMC article.
-
The First K+-Channel Blocker Described from Tityus fasciolatus Venom: The Purification, Molecular Cloning, and Functional Characterization of α-KTx4.9 (Tf5).Toxins (Basel). 2025 Feb 18;17(2):96. doi: 10.3390/toxins17020096. Toxins (Basel). 2025. PMID: 39998113 Free PMC article.
-
Structural and Functional Elucidation of Peptide Ts11 Shows Evidence of a Novel Subfamily of Scorpion Venom Toxins.Toxins (Basel). 2016 Sep 30;8(10):288. doi: 10.3390/toxins8100288. Toxins (Basel). 2016. PMID: 27706049 Free PMC article.
References
-
- Rodriguez de la Vega RC, Schwartz EF, Possani LD (2010) Mining on scorpion venom biodiversity. Toxicon 56: 1155–1161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

