Volatile anesthetics, not intravenous anesthetic propofol bind to and attenuate the activation of platelet receptor integrin αIIbβ3
- PMID: 23573252
- PMCID: PMC3616120
- DOI: 10.1371/journal.pone.0060415
Volatile anesthetics, not intravenous anesthetic propofol bind to and attenuate the activation of platelet receptor integrin αIIbβ3
Abstract
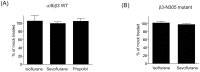
Background: In clinical reports, the usage of isoflurane and sevoflurane was associated with more surgical field bleeding in endoscopic sinus surgeries as compared to propofol. The activation of platelet receptor αIIbβ3 is a crucial event for platelet aggregation and clot stability. Here we studied the effect of isoflurane, sevoflurane, and propofol on the activation of αIIbβ3.
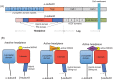
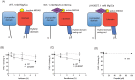
Methods: The effect of anesthetics on the activation of αIIbβ3 was probed using the activation sensitive antibody PAC-1 in both cell-based (platelets and αIIbβ3 transfectants) and cell-free assays. The binding sites of isoflurane on αIIbβ3 were explored using photoactivatable isoflurane (azi-isoflurane). The functional implication of revealed isoflurane binding sites were studied using alanine-scanning mutagenesis.
Results: Isoflurane and sevoflurane diminished the binding of PAC-1 to wild-type αIIbβ3 transfectants, but not to the high-affinity mutant, β3-N305T. Both anesthetics also impaired PAC-1 binding in a cell-free assay. In contrast, propofol did not affect the activation of αIIbβ3. Residues adducted by azi-isoflurane were near the calcium binding site (an important regulatory site termed SyMBS) just outside of the ligand binding site. The mutagenesis experiments demonstrated that these adducted residues were important in regulating integrin activation.
Conclusions: Isoflurane and sevoflurane, but not propofol, impaired the activation of αIIbβ3. Azi-isoflurane binds to the regulatory site of integrin αIIbβ3, thereby suggesting that isoflurane blocks ligand binding of αIIbβ3 in not a competitive, but an allosteric manner.
Conflict of interest statement
Figures





Similar articles
-
Propofol shares the binding site with isoflurane and sevoflurane on leukocyte function-associated antigen-1.Anesth Analg. 2013 Oct;117(4):803-811. doi: 10.1213/ANE.0b013e3182a00ae0. Epub 2013 Aug 19. Anesth Analg. 2013. PMID: 23960033 Free PMC article.
-
The in vitro effects of isoflurane, sevoflurane, and propofol on platelet aggregation.Anesth Analg. 1999 Feb;88(2):432-6. doi: 10.1097/00000539-199902000-00039. Anesth Analg. 1999. PMID: 9972770 Clinical Trial.
-
Volatile anesthetics affect macrophage phagocytosis.PLoS One. 2019 May 9;14(5):e0216163. doi: 10.1371/journal.pone.0216163. eCollection 2019. PLoS One. 2019. PMID: 31071106 Free PMC article.
-
[Sevoflurane and propofol: original and generic].Ann Fr Anesth Reanim. 2008 Jan;27(1):120-2. doi: 10.1016/j.annfar.2007.11.010. Epub 2008 Jan 8. Ann Fr Anesth Reanim. 2008. PMID: 18191934 Review. French. No abstract available.
-
Sevoflurane: an ideal agent for adult day-case anesthesia?Acta Anaesthesiol Scand. 2003 Sep;47(8):917-31. doi: 10.1034/j.1399-6576.2003.00196.x. Acta Anaesthesiol Scand. 2003. PMID: 12904182 Review.
Cited by
-
Surgical Site Infections and Perioperative Optimization of Host Immunity by Selection of Anesthetics.Biomed Res Int. 2021 Mar 8;2021:5576959. doi: 10.1155/2021/5576959. eCollection 2021. Biomed Res Int. 2021. PMID: 33763473 Free PMC article. Review.
-
Tirofiban, a Glycoprotein IIb/IIIa Antagonist, Has a Protective Effect on Decompression Sickness in Rats: Is the Crosstalk Between Platelet and Leukocytes Essential?Front Physiol. 2018 Jul 11;9:906. doi: 10.3389/fphys.2018.00906. eCollection 2018. Front Physiol. 2018. PMID: 30050468 Free PMC article.
-
Mechanisms revealed through general anesthetic photolabeling.Curr Anesthesiol Rep. 2014 Mar 1;4(1):57-66. doi: 10.1007/s40140-013-0040-7. Curr Anesthesiol Rep. 2014. PMID: 24563623 Free PMC article.
-
Pulmonary static inflation with 50% xenon attenuates decline in tissue factor in patients undergoing Stanford type A acute aortic dissection repair.J Thorac Dis. 2018 Jul;10(7):4368-4376. doi: 10.21037/jtd.2018.06.95. J Thorac Dis. 2018. PMID: 30174885 Free PMC article.
-
Effect of Inhalation Anesthetics on Tumor Metastasis.Technol Cancer Res Treat. 2022 Jan-Dec;21:15330338221121092. doi: 10.1177/15330338221121092. Technol Cancer Res Treat. 2022. PMID: 36131554 Free PMC article. Review.
References
-
- Franks NP (2008) General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 9: 370–386. - PubMed
-
- Ueda I (1971) The effects of volatile general anesthetics on adenosine diphosphate-induced platelet aggregation. Anesthesiology 34: 405–408. - PubMed
-
- Ahn HJ, Chung SK, Dhong HJ, Kim HY, Ahn JH, et al. (2008) Comparison of surgical conditions during propofol or sevoflurane anaesthesia for endoscopic sinus surgery. Br J Anaesth 100: 50–54. - PubMed
-
- Blackwell KE, Ross DA, Kapur P, Calcaterra TC (1993) Propofol for maintenance of general anesthesia: a technique to limit blood loss during endoscopic sinus surgery. Am J Otolaryngol 14: 262–266. - PubMed
-
- Eberhart LH, Folz BJ, Wulf H, Geldner G (2003) Intravenous anesthesia provides optimal surgical conditions during microscopic and endoscopic sinus surgery. Laryngoscope 113: 1369–1373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

