Haptoglobin phenotype, preeclampsia risk and the efficacy of vitamin C and E supplementation to prevent preeclampsia in a racially diverse population
- PMID: 23573260
- PMCID: PMC3616124
- DOI: 10.1371/journal.pone.0060479
Haptoglobin phenotype, preeclampsia risk and the efficacy of vitamin C and E supplementation to prevent preeclampsia in a racially diverse population
Abstract
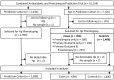
Haptoglobin's (Hp) antioxidant and pro-angiogenic properties differ between the 1-1, 2-1, and 2-2 phenotypes. Hp phenotype affects cardiovascular disease risk and treatment response to antioxidant vitamins in some non-pregnant populations. We previously demonstrated that preeclampsia risk was doubled in white Hp 2-1 women, compared to Hp 1-1 women. Our objectives were to determine whether we could reproduce this finding in a larger cohort, and to determine whether Hp phenotype influences lack of efficacy of antioxidant vitamins in preventing preeclampsia and serious complications of pregnancy-associated hypertension (PAH). This is a secondary analysis of a randomized controlled trial in which 10,154 low-risk women received daily vitamin C and E, or placebo, from 9-16 weeks gestation until delivery. Hp phenotype was determined in the study prediction cohort (n = 2,393) and a case-control cohort (703 cases, 1,406 controls). The primary outcome was severe PAH, or mild or severe PAH with elevated liver enzymes, elevated serum creatinine, thrombocytopenia, eclampsia, fetal growth restriction, medically indicated preterm birth or perinatal death. Preeclampsia was a secondary outcome. Odds ratios were estimated by logistic regression. Sampling weights were used to reduce bias from an overrepresentation of women with preeclampsia or the primary outcome. There was no relationship between Hp phenotype and the primary outcome or preeclampsia in Hispanic, white/other or black women. Vitamin supplementation did not reduce the risk of the primary outcome or preeclampsia in women of any phenotype. Supplementation increased preeclampsia risk (odds ratio 3.30; 95% confidence interval 1.61-6.82, p<0.01) in Hispanic Hp 2-2 women. Hp phenotype does not influence preeclampsia risk, or identify a subset of women who may benefit from vitamin C and E supplementation to prevent preeclampsia.
Conflict of interest statement
Figures

References
-
- Hauth JC, Ewell MG, Levine RJ, Esterlitz JR, Sibai B, et al. (2000) Pregnancy outcomes in healthy nulliparas who developed hypertension. Calcium for Preeclampsia Prevention Study Group. Obstet Gynecol 95: 24–28. - PubMed
-
- Knuist M, Bonsel GJ, Zondervan HA, Treffers PE (1998) Intensification of fetal and maternal surveillance in pregnant women with hypertensive disorders. Int J Gynaecol Obstet 61: 127–133. - PubMed
-
- World Health Organization (2005) World Health Report: Make Every Mother, and Child Count. Geneva: WHO.
-
- Altman D, Carroli G, Duley L, Farrell B, Moodley J, et al. (2002) Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet 359: 1877–1890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HD36801/HD/NICHD NIH HHS/United States
- UG1 HD027869/HD/NICHD NIH HHS/United States
- U10 HD034136/HD/NICHD NIH HHS/United States
- U10 HD040485/HD/NICHD NIH HHS/United States
- HD40485/HD/NICHD NIH HHS/United States
- HD40560/HD/NICHD NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- U10 HD040560/HD/NICHD NIH HHS/United States
- U01 HD036801/HD/NICHD NIH HHS/United States
- U10 HD053118/HD/NICHD NIH HHS/United States
- UL1 RR024989/RR/NCRR NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- U10 HD040500/HD/NICHD NIH HHS/United States
- P01 HD030367/HD/NICHD NIH HHS/United States
- U10 HD040544/HD/NICHD NIH HHS/United States
- UL1 RR024153/RR/NCRR NIH HHS/United States
- UG1 HD034116/HD/NICHD NIH HHS/United States
- HD27869/HD/NICHD NIH HHS/United States
- HD34136/HD/NICHD NIH HHS/United States
- UG1 HD040560/HD/NICHD NIH HHS/United States
- HD53118/HD/NICHD NIH HHS/United States
- K12 HD065987/HD/NICHD NIH HHS/United States
- UG1 HD053097/HD/NICHD NIH HHS/United States
- HD27860/HD/NICHD NIH HHS/United States
- UG1 HD027915/HD/NICHD NIH HHS/United States
- CAPMC/ CIHR/Canada
- HD40512/HD/NICHD NIH HHS/United States
- UG1 HD040544/HD/NICHD NIH HHS/United States
- UG1 HD034208/HD/NICHD NIH HHS/United States
- M01 RR00080/RR/NCRR NIH HHS/United States
- UG1 HD040512/HD/NICHD NIH HHS/United States
- HD40545/HD/NICHD NIH HHS/United States
- U10 HD034116/HD/NICHD NIH HHS/United States
- HD21410/HD/NICHD NIH HHS/United States
- U10 HD027869/HD/NICHD NIH HHS/United States
- U10 HD027917/HD/NICHD NIH HHS/United States
- HD34116/HD/NICHD NIH HHS/United States
- U10 HD027915/HD/NICHD NIH HHS/United States
- UG1 HD040545/HD/NICHD NIH HHS/United States
- UG1 HD040485/HD/NICHD NIH HHS/United States
- U10 HD027860/HD/NICHD NIH HHS/United States
- HD40500/HD/NICHD NIH HHS/United States
- U10 HD034208/HD/NICHD NIH HHS/United States
- U10 HD053097/HD/NICHD NIH HHS/United States
- HD34208/HD/NICHD NIH HHS/United States
- HD27915/HD/NICHD NIH HHS/United States
- UG1 HD040500/HD/NICHD NIH HHS/United States
- R24 HD050924/HD/NICHD NIH HHS/United States
- U10 HD040512/HD/NICHD NIH HHS/United States
- HD27917/HD/NICHD NIH HHS/United States
- U10 HD021410/HD/NICHD NIH HHS/United States
- M01 RR000080/RR/NCRR NIH HHS/United States
- U24 HD036801/HD/NICHD NIH HHS/United States
- U10 HD036801/HD/NICHD NIH HHS/United States
- HD53097/HD/NICHD NIH HHS/United States
- U10 HD040545/HD/NICHD NIH HHS/United States
- K12HD065987/HD/NICHD NIH HHS/United States
- HD40544/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

